Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.64 no.3 Porto Alegre Jul./Set. 2016
ORIGINAL / ORIGINAL
Effectiveness of photodynamic therapy on Candida species isolated from oral samples of children exposed and not exposed to HIV
Efetividade da terapia fotodinâmica sobre espécies de Candida isoladas de amostras bucais de crianças expostas e não expostas ao HIV
Francine Cristina da SILVAI; Luciano Pereira ROSAI; Antonio Luiz Barbosa PINHEIROII; Cristiane Yumi KOGA-ITOIII; Bruno Pereira de ARAÚJOI; Vivian de Oliveira VIANAI; Laíze Aparecida Nunes Lopes CAMPOSI
I Universidade Federal da Bahia, Instituto Multidisciplinar em Saúde
II Universidade Federal da Bahia, Faculdade de Odontologia, Departamento de Propedêutica e Clínica Integrada. Salvador, BA, Brasil
III Universidade Estadual Paulista Júlio de Mesquita Filho, Instituto de Ciência e Tecnologia, Departamento de Engenharia Ambiental. São José dos Campos, SP, Brasil
ABSTRACT
Objective
Identify yeast species isolated from unexposed, exposed and HIV-carrier children, and verify the effectiveness of low power laser photodynamic therapy (PDT) on the yeasts species belonging to the Candida genus.
Methods
Fifty children assisted by the Public Health Program of the city of Vitória da Conquista, Bahia, were selected and divided into three groups: unexposed to HIV, exposed to HIV during pregnancy, and HIV-carrier. Saliva samples were collected in a disposable sterile universal container and were plated to Sabouraud dextrose agar supplemented with 0.1 mg/mL chloramphenicol. The plates were incubated at 37°C for 48 h. Three strains of each patient were identified by using an API 20 C AUX system. The strains were submitted to photodynamic therapy (PDT) with a 660 nm low power laser and methylene blue dye at different times of irradiation (90, 180 and 282 sec.).
Results
The results showed that the most prevalent species was Candida albicans followed by Candida famata (second most prevalent in unexposed to HIV and HIV carriers)) and Candida parapsilosis (second most prevalent in exposed to HIV group). The CFU/mL of Candida spp. decreased significantly (p<0,05) in all groups treated with PDT compared to the controls. Photodynamic therapy treatments at different exposure times (e.g., PS+L90+, PS+L180+, PS+L282+) revealed that the exposure time of 282 sec. gave the highest reduction of the mean logarithmic CFU/mL.
Conclusion
Candida albicans was the most prevalent Candida species in these three groups and Candida non-albicans species, when combined, amounted to a significant percentage of Candida isolates. Photodynamic therapy was effective in inactivating the Candida spp. isolated from the oral cavity of children not exposed to HIV, exposed to HIV and HIV-carriers, with the best photodynamic therapy irradiation time being 282 sec.
Indexing terms: Candida. Children. HIV. Methylene blue. Photochemotherapy.
RESUMO
Objetivo
Identificar espécies de leveduras isoladas de crianças não expostas, expostas ao HIV e portadoras de AIDS, e verificar a eficácia da terapia fotodinâmica com laser de baixa potência sobre as espécies de leveduras pertencentes ao gênero Candida.
Métodos
Cinquenta crianças atendidas pelo Programa de Saúde Pública da cidade de Vitória da Conquista, Bahia, foram selecionadas e divididas em três grupos: não expostas ao HIV, expostas ao HIV durante a gravidez e portadoras da AIDS. Amostras de saliva foram coletadas em um recipiente universal descartável estéril e foram semeadas em ágar Sabouraud dextrose suplementado com 0,1 mg / mL de cloranfenicol. As placas foram incubadas a 37°C durante 48h. Três cepas de cada paciente foram identificadas utilizando o sistema API 20 C AUX. As cepas foram submetidas à Terapia Fotodinâmica com laser de baixa potência de 660 nm e corante azul de metileno em diferentes tempos de irradiação (90, 180 e 282 segundos).
Resultados
Os resultados mostraram que a espécie isolada mais prevalente nos grupos estuddos foi Candida albicans, seguida de Candida famata (segunda mais prevalene nos grupos não expostos ao HIV e com AIDS) e Candida parapsilosis (segunda mais prevalente no grupo exposto ao HIV). Houve diminuição significante de CFU/ml de Candida spp. (p <0,05) em todos os grupos tratados com terapia fotodinâmica, em comparação com os controles. A terapia fotodinâmica, nos diferentes tempos (e.g., PS+L90+, PS+L180+, PS+L282+) mostrou que o tempo de 282 seg. apresentou a maior redução em media de logarítmo de UFC/mL.
Conclusão
Candida albicans foi a espécie de Candida mais prevalente isolada nos três grupos e as espécies de Candida não-albicans, quando combinadas, contribuíram com porcentagem significativa dos isolados de Candida. A PDT foi eficaz na inativação de Candida spp. isoladas a partir da cavidade oral de crianças não expostas, expostas ao HIV e portadoras da AIDS, com o melhor tempo de irradiação sendo o de 282 seg.
Termos de indexação: Candida. Criança. HIV. Azul de metileno. Fotoquimioterapia.
INTRODUCTION
Acquired Immunodeficiency syndrome (AIDS) has become a global epidemic that has transformed medical practices as well as public health policies and is currently undergoing an epidemiological transition with the virus now spreading to small urban centers, heterosexual individuals, and vertical transmission1. Human Immunodeficiency virus (HIV) is a retrovirus that has a predilection for immune cells, especially TCD4 lymphocytes and causes their progressive destruction. Patients with AIDS are more susceptible to the so-called "opportunistic infections"2-3. Since the virus causes substantial immunosuppression, pathogens can easily multiply, which in turn increases the chances of infection, thus leading to increased morbidity and hospitalization time4.
In AIDS patients, among the various opportunistic infections, oral candidiasis is one of the most prevalent. The yeasts of Candida genus, which constitute a large population of the oral microbiota, play an important role because they are favored by antibiotic substances, which is common in immunosuppressed patients5. Infection frequency and severity resulting from the manifestation of opportunistic Candida spp. in AIDS patients increase with the progression of the disease because of a weak of defense system due to the lack of T lymphocytes, macrophages and dendritic cells6. Although the dissemination of antiretroviral drugs has led to a decrease in the frequency of opportunistic infections, the occurrence of many adverse reactions, delayed diagnosis and non-responsiveness to therapy, candidiasis is still prevalent and clinically debilitating7. Among children infected with HIV, both in developed and in developing countries, pseudomembranous candidiasis is the most prevalent form of the disease, followed by erythematous candidiasis and angular cheilitis. HIVpositive children with oral candidiasis have poor survival prognosis, besides the fact that the disease may worsen the condition of the already compromised immune system as colonization may sprend to the esophagus and the respiratory tract, hampering feeding, leading to malnutrition and accentuating immunosuppression8-9.
The treatment of superficial fungal infections using photosensitizing agents and light, known as photodynamic therapy (PDT), has gathered attention as a viable alternative over conventional treatments, because these microorganisms are becoming increasingly resistant to the effects of antibiotics and antifungal agents10-13. PDT is based on the principle that the interaction of light at a suitable wavelength with a non-toxic compound (photosensitizer) in the presence of oxygen results in the formation of reactive oxygen species such as singlet oxygen (¹O2) and toxic radicals. These radicals are capable of irreversibly damaging cellular structures, which kill microorganisms by fast oxidation of its constituents, especially membrane macromolecules3-13.
Another advantage of PDT treatment is the specificity of the photosensitizer to the microorganism and the absence of harmful effects on non-target cells such as the host cells. The combination of a photosensitizing dye with low-intensity light does not cause damage to animal cells, because the photosensitizer concentrations differ in addition to higher doses of light being required12-14.
The advantages of PDT as a treatment include no side effects, easy applicability, verified anti-inflammatory and analgesic effects, and low costs, which may contribute to the improvement of the patients quality of life, especially those who are subjected to painful or invasive treatments that are cost-intensive.
Therefore, this study aimed to isolate and identify Candida genus yeasts obtained from oral samples of unexposed, exposed and HIV-carrier children, in collaboration with the National Health System (Vitória da Conquista, Bahia, Brazil) and to evaluate the activity of PDT using a low power laser and methylene blue dye on the identified Candida spp.
METHODS
The protocol used in this study was approved by the Committee of Ethics in Research of the Department of Health of Bahia - SESAB in Salvador-Brazil (registration number: CAAE 0124.0.053.000-08, number opinion: 348.2009).
Patients selection
For this study, 50 children assisted by the Public Health Program of the city of Vitória da Conquista, Bahia, were selected and divided into three groups: (a) children unexposed to HIV (n = 20); (b) children exposed to HIV during pregnancy, but who did not contract the virus through vertical transmission (n = 15); and (c) HIV-carrier children (n = 15).
The inclusion criteria were: children aged 5-12 years; positive for Candida spp. in oral samples; parents and / or guardians authorizing the participation by signing the informed consent. The exclusion criteria were: antibiotic or antifungal therapy for at least 60 days before collection; Metabolically controlled diabetic individuals; patients with orthodontic braces (top or bottom); and presence of lesions suggestive of oral candidiasis.
Patients were included in the group of children with positive status for AIDS if they were HIV-seropositive who made use of antiretroviral medications for at least 1 year. For all patients, general and oral health conditions, drugs association (e.g. "cocktail") and treatment time were evaluated during the medical interview.
Collection and Samples processing
After obtaining informed consent from the parents of the children, saliva samples were collected in a disposable sterile universal container. The containers were identified by code and kept in a thermal bag with ice for transportation to the Microbiology laboratory of the Multidisciplinary Institute of Health, Federal University of Bahia, Campus Vitória da Conquista, where sample processing was conducted within a maximum period of 3 hours from sample collection and processing.
Decimal dilutions (10-1, 10-2, and 10-3) of the collected saliva samples were made with sterile saline solution (0.85% NaCl) using eppendorf tubes. An aliquot (100 mL) of pure saliva and dilutions were plated in duplicate on Petri dishes containing Sabouraud dextrose agar (Himedia, Mumbai, India) supplemented with 0.1 mg/ mL chloramphenicol (Union Nacional SA Pharmaceutical Chemistry). The plates were incubated at 37°C for 48 h. Once growth stopped, the plates were incubated at room temperature for 5 days.
After growth, colonies were examined for morphological characteristics (e.g., size, shape and surface) and counted to obtain the colony forming units per milliliter (CFU/mL). Subsequently, isolates were obtained from three strains suggestive of yeast randomly selected from each individual sample, plated on Sabouraud dextrose agar and incubated for 24 h at 37°C. After growth, the plates were stored for subsequent identification.
Samples identification
Three strains were identified among the isolates obtained from each patient using an API 20 C AUX system (bioMerieux® AS L'Etoile, France). The kit consists of a gallery that include 20 domes containing dehydrated substrates to perform 19 sugars assimilation tests. The domes were inoculated with minimal culture medium and yeasts developed only if they were capable of using the corresponding substrate. Evaluation of these reactions was made by comparing the growth in the different substrates and the strains were identified by querying the analytical catalogue or using an identification system based on a numerical profile.
Photosensitizing dye and light parameters
Methylene blue (Sigma-Aldrich Corp., St. Louis - USA) was diluted at a concentration of 0.1 mg/mL in sterile distilled water and then filtered on a syringecoupled filter membrane (pore size, 0.22 μm) (Millipore, São Paulo, Brazil).
Irradiation with a low power laser was carried out using a PhotonLase III apparatus (DMC Equipment LTDA, São Carlos-SP, Brazil) equipped with the active medium InGaAIP at a wavelength of 660 nm, which produces red visible light corresponding to the maximum absorption length of the methylene blue dye. Other parameters were output power of 100 mW, beam diameter of 0.028 cm2, continuous application mode and irradiation times of 90, 180, and 282 sec., which resulted in total energy of 9 J, 18 J, and 28,2 J and fluences of 321 J/cm2, 637 J/cm2 and 999 J/cm2, respectively. The laser beam output was kept at the distance of 2 mm from the microorganism suspension, resulting in irradiance of 3.571 mW/cm2.
Experimental conditions
After 24 h culture of the identified species, a 1 x 106 cells/mL standardized suspension was obtained by spectrophotometry. In a dark room, 100 μL of the standard microorganism suspension and 100 μL methylene blue, were added to the plates wells in a laminar flow hood and left in the dark for 5 minutes (pre-irradiation or dye impregnation to the cells phase). Then, irradiation was performed for 90 sec., 180 sec., and 282 sec. (PS+L90+, PS+L180+, and PS+L282+, respectively) using a low power laser. After serial irradiation dilutions (e.g., 10-1, 10-2, and 10-3) cultures were plated in duplicate on Petri dishes containing Sabouraud dextrose agar (Himedia, Mumbai, India) and incubated for 24 h at 37°C. After the incubation period, CFU/mL count was performed. Samples not subjected to any treatment and containing 100 μL of sterile saline were used as controls. The control groups were only subjected to the effect of the dye for 5 min (PS+ L-) or were only irradiated for 90 sec., 180 sec., and 282 sec. (PS-L90+, PS-L180+ and PS-L282+, respectively).
Statistical analysis of results
The isolated yeasts were identified and the data expressed as percentage of yeasts observed in each group. To observe the effectiveness of PDT against Candida spp. and obtain a comparisons with other treatments, the mean logarithm of CFU/mL for each experimental condition (e.g., PS-L-; PS+L-; PS-L90+; PSL180+; PS-L282+; PS+L90+; PS+L180+; and PS+L282+) was calculated for each individual. The Kolmogorov Smirnov normality test was used to observe the sample normal distribution. A one-way analysis of variance (ANOVA) was used to compare the experimental treatments within the same group and the mean logarithm of CFU/mL after PDT treatments for different time points between groups. P-values < 0.05 were considered statistically significant.
RESULTS
Table 1 shows the percentages of each yeast species identified in all the groups of children selected for the study. The most prevalent species was Candida albicans. Only the yeasts belonging to the Candida genus were used in our experiments.
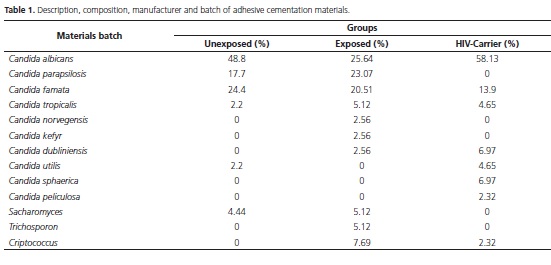
For the group of unexposed children, the experimental treatments were effective in reducing Candida spp. CFU/mL, with the exception of the groups PS+L- and PS-L90+, which presented no statistical difference (p > 0.05) when compared to the control group (PS-L) (Figure 1). No statistical difference was found between the groups treated with photosensitizing dye only (PS+L-) and those treated only with laser application at different times (PS-L90+, PS-L180+, and PS-L282+) (p > 0.05), indicating the same treatment effectiveness. The groups treated with PDT (PS+L+) revealed the best results in reduction of the mean logarithmic CFU/mL of Candida spp. at all exposure times, and a significant difference (p < 0.05) was found compared to the control groups (PS+Land PS- L90+, PS-L180+, PS-L282+). PDT treatments at different exposure times (e.g., PS+L90+, PS+L180+, PS+L282+) revealed that the exposure time of 282 sec. gave the highest reduction of the mean logarithmic CFU/ mL.
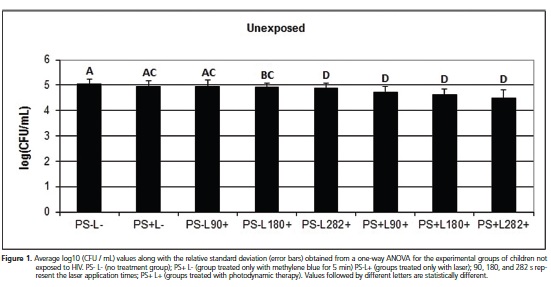
Different results were obtained from the data analysis of the HIV-exposed children group, for which all treatments showed significant reduction (p < 0.05) in the mean logarithmic CFU/mL count when compared to the control. The comparison between the groups treated with methylene blue only (PS+ L-) or with laser only at all the exposure times (e.g., PS- L90+, PS- L180+ and PS- L282+) and the groups treated with PDT at all the exposure times (e.g., PS+L90+, PS+L180+, and PS+L282+) showed statistically significant differences (p < 0.05), with PDT treatment showing the highest reduction in CFU count. Moreover, there was no statistically significant difference in the CFU count reduction between the treatments with laser only at all the exposure times (e.g., PS-L90+, PSL180+ and PS-L282+). However, when the CFU count were compared between the groups treated with PDT, it was observed that exposure time of 282 sec. gave the best result. This was in agreement with the results obtained for the groups of children unexposed to HIV (Figure 2).
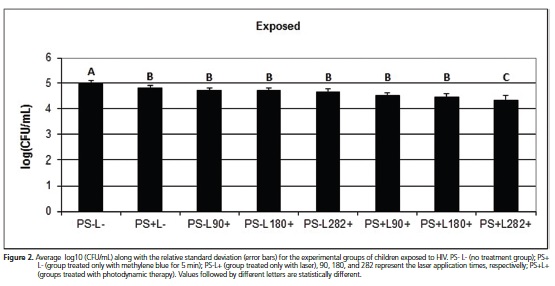
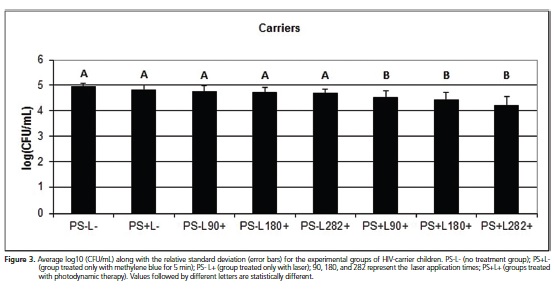
For the group of HIV-carrier children, all experimental treatments showed antifungal activity against Candida spp., differing from the control group (p < 0.05). For the groups treated with methylene blue or laser only, there was no statistical difference, demonstrating equal effectiveness in reducing the CFU count of Candida.spp. Tests conducted with PDT showed the best results in Candida spp. inactivation compared to the other groups, with the best reduction observed in the test with PDT treatment for 282 sec., which was similar to the results obtained for the groups of children exposed and unexposed to HIV (Figure 3).
DISCUSSION
Candida albicans is considered the main causative agent of oral candidiasis in humans, especially in HIV-positive patients. Other Candida species may colonize the oral cavity and become pathogenic mainly in immunocompromised conditions. Another concern relateding to such infections stems from the fact that yeasts are growing increasingly resistant to commercial antifungal agents15-17. In the present study, Candida albicans was found to be the most prevalent species in the three groups tested, which corroborates with the results of Sant'Ana et al.18 who verified the prevalence of Candida species isolated from AIDS patients and their resistance to fluconazole, itraconazole and ketoconazole used in the treatment of oral candidiasis. Candida albicans was identified in 91% of the obtained isolates, and the remaining 9% was identified as other Candida species, with half of them presenting resistance to the drugs tested. Similarly, Costa et al.6 found that Candida albicans was the most prevalent species in the oral cavity of HIV-infected patients; however, this was not related to low TCD4 cell counts or high viral replication levels. The presence of pathogenic Candida species other than Candida albicans is associated with worst prognosis of candidiasis because, in normal immunity, these species are not related to infections but are most often recognized as constituents of the natural microbiota19.
Considering the increasing resistance of microorganisms to antifungal drugs and the high incidence of adverse reactions to therapeutics, which are particularly harmful in immunocompromised patients, it is necessary to develop alternative therapies for oral candidiasis that would also bring more comfort and be more effective in combating proliferation of pathogens. In several studies PDT has emerged as a viable alternative in the inactivation of pathogenic microrganisms12-13,16-20. Souza et al.21 investigated the effectiveness of PDT against Candida albicans in combination with the use of photosensitizing dyes such as methylene blue, toluidine blue, malachite green and isolated low-intensity laser light irradiation. In that report, the authors verified treatment efficacy by monitoring the reduction of yeast CFU/mL compared to colonies that were not subjected to any treatment. The efficacy of PDT was dependent on the energy density of the light used and the combination of laser light with methylene blue was more effective than laser irradiation alone, corroborating the results found in this study, in which PDT was more effective in reducing the CFU/mL count than laser irradiation alone at all the irradiation periods tested. Moreover, the greatest effectiveness was observed at the maximum time tested (e.g., 282 sec.).
Souza et al.22 found that laser irradiation and methylene blue used in isolation were not effective in reducing the growth of yeasts with the exception of the species Candida tropicalis, while their association caused formation of reactive oxygen species such as 1O2, which causes disruption to the microbial cell structure. Munin et al.23 concluded that the combination of laser and methylene blue was effective in reducing the production of Candida albicans germ tube, an essential virulence stage, after culturing these microorganisms in goat serum. Rossoni et al.16 proved that PDT associated with low power laser and methylene blue was effective to Candida albicans serotype A and B, although the samples from serotype B were more susceptible to PDT. The results of these studies are similar to those found in the analysis of the treatment effectiveness on the Candida spp. samples obtained from the three groups in the present study, where the combination of the photosensitizing dye and laser light association was more effective in reducing the number of CFU/mL with statistically significant differences compared to the control samples that were not subjected to any treatment.
Few studies investigated the efficiency of low power laser irradiation used alone. Basso et al.24 have tested the efficacy of laser in 5 J/cm², 10 J/cm², and 20 J/cm² doses on biofilm formed by Streptococcus mutans, Candida albicans, or both, showing microorganism inhibition. This can be explained with the fact that laser alone does not generate reactive oxygen species, which destabilize cell membrane by oxidizing its molecules and causing cell death, a property that makes PDT treatment more effective. In this study it was found that laser irradiation alone inactivated microorganisms, but unlike the other treatment groups, in the group of unexposed children, this result was not statistically significant at the irradiation time of 90 sec. However, PDT showed better results even in those groups for which laser irradiation was effective. According to Lavi et al.25, compounds naturally present in the cell membrane may generate free radicals through activation by light, being this one possible explanation for the effectiveness that laser irradiation alone showed in some treatments; however, additional studies are necessary to confirm this hypothesis.
According to Denis et al.26 and Wainwright13, despite the natural antimicrobial properties of methylene blue, in order to generate compounds such as 1O2 that are capable of destroying the cells to which the treatment is directed, it needs to be energized to an excited state by light at an appropriate wavelength. In children unexposed to HIV, the treatment only with the photosensitizing did not show CFU/mL count reduction, while in the other treatments, PDT proved to be more effective with statistical difference compared to the control group.
Many studies aiming to prove the efficacy of PDT involved animal models. Martins et al.27 evaluated the effects of PDT on the pathogenicity of Candida albicans in the dorsum of the tongue of rats. After candidiasis development, animals were submitted to methylene blue and laser treatments, laser only, methylene blue only and saline only. In animals subjected to PDT, greater lesion reduction as well as the reduction of phospholipases and proteinases from the colonies was found. Similar results were obtained by Junqueira et al.28 who, by microscopic analysis of the tongue of 72 rats subjected to the same conditions, demonstrated lower rates of lesions, as well as lower inflammatory response in animals that had undergone PDT. The results of these studies indicated the potential efficacy of in vivo treatment of lesions caused by microorganisms, following the tendency of all in vitro studies that have also been performed. According to Gonzales et al.15, fungal cells may be extensively eliminated by PDT using laser doses at rates below those required to cause damage to keratinocytes, indicating that such treatment has a broad therapeutic window.
Another interesting finding was presented in the study performed by Scwingel et al.3, who showed a similar effectiveness of methylene blue, fluconazole, and PDT combined with low power laser in the in vivo treatment of oral candidiasis; only PDT was abel to prevent lesion recurrence, even after 30 days, compared with the conventional therapy using fluconazole. This study also demonstrated the effectiveness of PDT and provides additional information that was not observed in other studies, that is, its ability to inhibit lesion recurrence by Candida spp., thus suggesting a probable residual effect.
These findings proved the effectiveness of PDT as a treatment capable of reducing the damage caused by candidiasis and other infections. The existence of this new therapeutic alternative, associated with good oral hygiene and treatments against HIV, could considerably contribute to improving the quality of life of patients susceptible to opportunistic infections20.
Therefore, we conclude that PDT was effective in inactivating Candida spp. isolated from the oral cavity of children not exposed to HIV, exposed to HIV and HIVcarriers, with the best PDT irradiation time being 282 sec. Furthermore, these results indicate that PDT has potential as an alternative therapy and used alone or as an adjuvant to HIV-positive individuals who have already undergone treatments with antibiotics and antifungals.
ACKNOWLEDGEMENTS
This work was supported by the Foundation for Research Support of the State of Bahia (grant term SUS0039/2009 and APP 0091/2009). We thank the patients and to their caregivers for their collaboration as research participants.
Collaborators
FC SILVA, project design and coordination thereof. LP ROSA, project design, statistics and analysis of results. ALB PINHEIRO, technical advice on the laser. CYK ITO, technical consulting in microbiology. BP ARAUJO, execution of experiments and preparation of the article. VO VIANA, execution of experiments and preparation of the article. LAN CAMPOS, execution of experiments and preparation of the article.
REFERENCES
1. Soares VYR, Lúcio Filho CEP, Carvalho LIM, Silva AMMM, Eulálio KD. Clinical and epidemiological analysis of patients with HIV/ AIDS admitted to a reference hospital in the northeast region of Brazil. Rev Inst Med Trop São Paulo. 2008;50(6):327-32. doi: 10.1590/S0036-46652008000600003 [ Links ]
2. Agwu E, Ihongbe JC, McManus BA, Moran GP, Coleman DC, Sullivan DJ. Distribution of yeast species associated with oral lesions in HIV-infected patients in Southwest Uganda. Med Mycol. 2012;50(3):276-80. doi: 10.3109/13693786.2011.604862
3. Scwingel AR, Barcessat AR, Núñez SC, Ribeiro MS. Antimicrobial photodynamic therapy in the treatment of oral candidiasis in HIV-infected patients. Photomed Laser Surg. 2012;30(8):429- 32. doi: 10.1089/pho.2012.3225
4. Chakraborty N, Mukhrrjee E, Santra S, Sarkar RN, Banerjee D, Guha SK, et al. Current trends of opportunistic infections among HIV-seropositive patients from eastern Indian. Jpn J Infect Dis. 2008;61(1):49-53.
5. Ribeiro EL, Scroferneker ML, Cavalhaes MS, Campos CC, Nagato GM, Souza NA, et al. Phenotypic aspects of oral strains of Candida albicans in children with down's syndrome. Braz J Biol. 2006;66(3):939-944. doi: 10.1590/S1519- 69842006000500020
6. Costa CR, Cohen AJ, Fernandes OFL, Miranda KC, Passos XS, Souza LKH, et al. Asymptomatic oral carriage of Candida species in HIV-infected patients in the highly active antiretroviral therapy era. Rev Inst Med Trop São Paulo. 2006;48(5):257- 261. doi: 10.1590/S0036-46652006000500004
7. Wingeter MA, Guilhermetti E, Shinobu CS, Takaki I, Svidzinski TIE. Identificação microbiológica e sensibilidade in vitro de Candida isoladas da cavidade oral de indivíduos HIV positivos. Rev Soc Bras Med Trop. 2007;40(3):272-276. doi: 10.1590/ S0037-86822007000300004
8. Leão JC, Ribeiro CMB, Carvalho AAT, Frezzini C, Porter S. Oral complications of HIV disease. Clinics. 2009;64(5):459-470. doi: 10.1590/S1807-59322009000500014
9. Guerra LM, Pereira AC, Hebling E, Meneghim MC. Manifestações bucais da AIDS em crianças: implicações clínicas para o cirurgião-dentista. Rev Odontol Univ Cid São Paulo. 2007;19(1):77-83.
10. Sardi JC, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJ. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013;62(Pt1):10-24. doi: 10.1099/jmm.0.045054-0
11. Lyon JP, Rezende RR, Rabelo MP, de Lima CJ, Moreira LM. Synergic effect of photodynamic therapy with methylene blue and surfactants in the inhibition of Candida albicans. Mycopathologia. 2013;175(1):159-64. doi: 10.1007/s11046- 012-9601-4
12. Wainwright M. 'Safe' photoantimicrobials for skin and softtissue infections. Int J Antimicrob Agents. 2010;36(1):14-8. doi:10.1016/j.ijantimicag.2010.03.002
13. Wainwright M. Methylene blue derivatives--suitable photoantimicrobials for blood product disinfection? Int J Antimicrob Agents. 2000;16(4):381-94. doi: 10.1016/S0924- 8579(00)00207-7
14. Caminos DA, Durantini EN. Photodynamic inactivation of Escherichia coli immobilized on agar surfaces by a tricationic porphyrin. Bioorg Med Chem. 2006;14(12):4253-9. doi: 10.1016/j.bmc.2006.01.058
15. Gonzales FP, Maisch T. Photodynamic inactivation for controlling Candida albicans infections. Fungal Biol. 2012;116(1):1-10. doi: 10.1016/j.funbio.2011.10.001
16. Rossoni RD, Barbosa JO, Oliveira FE, Oliveira LD, Jorge AO, Junqueira JC. Biofilms of Candida albicans serotypes A and B differ in their sensitivity to photodynamic therapy. Lasers Med Sci. 2014;29(5):1679-84. doi: 10.1007/s10103-014-1570-z
17. Silva MP, Santos TA, Barros PP, Camargo Ribeiro F, Junqueira JC, Jorge AO. Action of antimicrobial photodynamic therapy on heterotypic biofilm: Candida albicans and Bacillus atrophaeus. Lasers Med Sci. 2016;31(4):605-10 doi: 10.1007/s10103-016- 1876-0
18. Sant'Ana PL, Milan EP, Martinez R, Queiroz-Telles F, Ferreira MS, Alcântara AP, et al. Multicenter Brazilian study of oral Candida species isolated from Aids patients. Mem Inst Oswaldo Cruz. 2002. 97(2):253-257. doi: 10.1590/S0074- 02762002000200019
19. Chavasco JK, Paula CR, Hirata MH, Aleva NA, Melo CE, Gambale W, et al. Molecular identification of Candida dubliniensis isolated from oral lesions of HIV-positive and HIV-negative patients in São Paulo, Brazil. Rev Inst Med Trop São Paulo. 2006;48(1): 21-26. doi: 10.1590/S0036-46652006000100005
20. Javed F, Samaranayake LP, Romanos GE. Treatment of oral fungal infections using antimicrobial photodynamic therapy: a systematic review of currently available evidence. Photochem Photobiol Sci. 2014;13(5):726-34. doi: 10.1039/c3pp50426c
21. Souza RC, Junqueira JC, Rossoni RD, Pereira CA, Munin E, Jorge AO. Comparison of the photodynamic fungicidal efficacy of methylene blue, toluidine blue, malachite green and lowpower laser irradiation alone against Candida albicans. Lasers Med Sci. 2010;25(3):385-389. doi: 10.1007/s10103-009- 0706-z
22. Souza SC, Junqueira JC, Balducci I, Koga-Ito CY, Munin E, Jorge AO. Photosensitization of different Candida species by low power laser light. J Photochem Photobiol B. 2006;83(1):34-38. doi: 10.1016/j.jphotobiol.2005.12.002
23. Munin E, Giroldo LM, Alves LP, Costa MS. Study of germ tube formation by Candida albicans after photodynamic antimicrobial chemotherapy (PACT). J Photochem Photobiol B. 2007;88(1):16-20. doi: 10.1016/j.jphotobiol.2007.04.011
24. Basso FG, Oliveira CF, Fontana A, Kurachi C, Bagnato VS, Spolidório DM, et al. In vitro effect of low-level laser therapy on typical oral microbial biofilms. Braz Dent J. 2011;22(6):502- 510. doi: 10.1590/S0103-64402011000600011
25. Lavi R, Ankri R, Sinyakov M, Eichler M, Friedmann H, Shainberg A, et al. The plasma membrane is involved in the visible lighttissue interaction. Photomed Laser Surg. 2012;30(1):14-19. doi: 10.1089/pho.2011.3083
26. Denis TGS, Dai T, Izikson L, Astrakas C, Anderson RR, Hamblin MR, et al. All you need is light: Antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence. 2011;2(6):509-520. doi: 10.4161/viru.2.6.17889
27. Martins JS, Junqueira JC, Faria RL. Antimicrobial photodynamic therapy in rat experimental candidiasis: evaluation of pathogenicity factors of Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(1):71-77. doi: 10.1016/j.tripleo.2010.08.012
28. Junqueira JC, Martins JS, Faria RL, Colombo CE, Jorge AO. Photodynamic therapy for the treatment of buccal candidiasis in rats. Lasers Med Sci. 2009;24(6):877-84. doi: 10.1007/ s10103-009-0673-4
 Correspondence to:
Correspondence to:
FC SILVA
e-mail: drfransilva@yahoo.com.br
Received on: 25/5/2015
Final version resubmitted on: 28/6/2016
Approved on: 18/7/2016













