Serviços Personalizados
Artigo
Links relacionados
Compartilhar
IJD. International Journal of Dentistry
versão On-line ISSN 1806-146X
IJD, Int. j. dent. vol.9 no.1 Recife Jan./Mar. 2010
ORIGINAL ARTICLE ARTIGO ORIGINAL
Erosion-like lesions progression in human and bovine enamel
Progessão de lesões erosivas em substrato humano e bovino
Francisco Carlos Rehder NetoI; Cecília Pedroso TurssiII; Mônica Campos SerraIII
IDDS, MS, doctoral student, Department of Restorative Dentistry, School of Dentistry of Ribeirão Preto, University of São Paulo, Ribeirão Preto, São Paulo, Brazil
IIDDS, MS, PhD, Laboratory of Biomaterials Research, School of Dentistry, University of Uberaba (UNIUBE), Uberaba, Minas Gerais, Brazil
IIIDDS, MS, PhD, associate professor, Department of Restorative Dentistry, School of Dentistry of Ribeirão Preto, University of São Paulo, Ribeirão Preto, São Paulo, Brazil
ABSTRACT
Although on dental erosion studies bovine enamel has been considered a feasible option to human enamel, time dependence related to the erosive challenge remains uncertain. Thus, in this study the following null hypothesis was tested: 1) independent of the number of erosive challenges, no microhardness (SMH) differences exist between human and bovine enamel and 2) the behavior of bovine substrate along the erosive challenges do not differ from the human substrate. Human and bovine enamel slabs were embedded in epoxy resin and serially polished and flattened. Initial SMH tests were measured. The erosive challenge consists of an immersion of the specimens in continuously stirred orange juice (20ml) for 5 minutes at room temperature twice a day, for 5 days. Between the immersions the specimens were washed with deionized water and stored in artificial saliva (20ml). SMH tests were daily measured at the end of 2,4,6,8 and 10 challenges. Data were analyzed by two-way ANOVA as a split-plot design in time and Tukey's test. Regression analyses were used to model the SMH of human and bovine enamel over time. The first null hypothesis was rejected. Regression analysis exhibits a quadratic polynomial reduction of SMH in function of the number of erosive challenges for both substrates, revealing the admission of the second null hypothesis. Bovine substrate showed the most susceptible substrate to erosive challenge, but after several days of erosive challenge this difference disappears. Thus, the use of bovine tooth instead of human tooth in studies of erosive lesions formation seems to be feasible.
Key words: Erosion; microhardness; human enamel; bovine enamel.
RESUMO
Embora em estudos de erosão, o emprego do esmalte bovino seja considerado uma opção viável ao esmalte humano, a comparação do comportamento da indução das lesões erosivas nos diferentes substratos em relação à progressão/perda mineral permanece incerto. O objetivo deste estudo foi definir in vitro, a partir de um protocolo de indução de desgaste corrosivo, o tempo para formação de lesões cujo padrão, avaliado através de microdureza Knoop, seja semelhante em substratos bovinos e humanos. Fragmentos esmalte humano e bovino foram incluídos em resina epóxica, planificados, polidos. Um protocolo para o desenvolvimento de lesões erosivas foi instituído e cada ciclo erosivo consistiu da imersão dos espécimes em 20 ml de suco de laranja, em temperatura ambiente, por 5 minutos, sob agitação, duas vezes ao dia, durante 5 dias. No período entre as exposições e durante a noite, os espécimes foram mantidos em 20 ml de saliva artificial. Após o desafio erosivo leituras de microdureza foram efetuadas para avaliar a perda de conteúdo mineral. A analise de variância mostrou efeito significativo na interação substrato -tempo (p,0096). Ao longo dos episódios erosivos, os substratos dentais - humano e bovino - apresentaram um comportamento semelhante. A microdureza para ambos os substratos variou de acordo com uma função polinomial de segundo grau onde apesar de inicialmente não existir diferença entre os valores de microdureza dos substratos, o esmalte bovino se mostrou mais susceptível aos episódios erosivos; porém com o aumento do número de desafios, a diferença entre os substratos desapareceu.
Palavras-chave: Erosão dental; microdureza; esmalte humano; esmalte bovino
INTRODUCTION
There have been substantial advances in understanding the pathophysiology of dental erosion, especially through controlled in vitro studies. Regarding the tooth wear, the use of in vitro studies showed some advantages, like the high level of scientific control, ethical aspects related to certain experiments, reduction in experimental variability, the use of highly sensitive assessment methods, and the possibility of separate the components of tooth wear and investigate them separately.
Erosion-like lesions have been produced through different models, using both human1-5 and bovine teeth 1,5,f6,7. Bovine substrate is widely used in dental research and presents similarities with human enamel such as the chemical composition8, crystal orientations9 and microhardness10,11. Bovine teeth have some advantages in relation to the ethical aspects, being easier to obtain and manipulate than human teeth10. In fact, ethics committees are encouraging their use as an alternative for human substrate12. However, bovine enamel cannot be replaced for human enamel without validation8.
Although bovine enamel has been considered a feasible option to human enamel, few studies have compared artificial erosion lesions progression in both substrates1,5,6. The aim of this study was to evaluate whether an erosive challenge protocol that simulates acidic drinks intake is able to induce similar erosion-like lesions in human and bovine enamel.
MATERIAL AND METHODS
Experimental design
This study was designed as a randomized complete block with 15 replications and a split-plot factorial arrangement. The factors under study were: dental substrate at two levels (bovine and human enamel) and number of erosive challenges, at six levels, repeatedly measured on the same specimen, at different times (T0, T1, T2, T3, T4, and T5). The levels of the factor dental substrate were assigned to the main plot and the factor number of erosive challenges were assigned to the subplots. The following null hypothesis were tested: 1) independent of the number of erosive challenges, no microhardness (SMH) differences exist between human and bovine enamel and 2) the behavior of bovine substrate along the erosive challenges do not differ from human substrate. The response variable was surface microhardness (SMH).
Preparation of specimens
Sound third human molars and bovine incisors were cleaned to remove tissue remnants and stored in a 0.1% thymol solution, at 4°C. Each tooth was sectioned using a low-speed water-cooled diamond saw (Isomet 1000, Buehler, Lake Bluff, IL, USA) in order to obtain enamel slabs (3 x 2 x 2 mm) that were inspected for defects under a stereomicroscope at 40x magnification and discarded if pitted or cracked. The preselected slabs were embedded in epoxy resin (Epoxicure Resin, Buehler) with enamel surface facing up and flattened in a water-cooled polishing machine (Beta Grinder-Polisher, Buehler) using 400, 600 and 1200-grit Al2O3 papers, and polished on cloths with a 0.3 m.m alumina suspension (Alpha and Gamma Micropolish, Buehler, Lake Bluff, IL, USA). Specimens were ultrasonically cleaned in deionized water (T1440D, Odontobrás, Ribeirão Preto, SP, Brazil) for 10 minutes and stored at 37º C in 100% relative humidity.
Baseline microhardness measurements
Specimens of human and bovine enamel were tested for surface Knoop microhardness (HMV-2, Shimadzu, Kyoto, Japan), under an load of 25 g, applied for 30 s. Indentations were performed in each specimen at 150, 500 and 1500 u.m from superior margin, and at 100 u.m to the lateral margin. The microhardness was measured with the aid of dedicated software (New Age, Software Cams, South Ampton, PA, USA) and the average of surface microhardness (SMH) values was calculated. Specimens with microhardness values 20% above or 20% below the average value were discarded.
Erosive Challenges
The erosive challenge was based on that proposed by Amaechi et al, (1999). Briefly, the protocol consisted of immersing the specimens in 20 mL of pure ready to drink orange juice (pH - 3.74) (Fazenda Bela Vista, Tapiratiba, SP, Brazil) in an erlenmeyer flask, which was then placed in an orbital shaker (CT155, Cientec, Piracicaba, SP, Brazil), with stirring velocity of 100 rpm for 5 minutes, at the room temperature (25°C), twice daily, for 5 days. From one immersion to the other, the specimens were washed with deionized water and stored in 20 mL of the artificial saliva (pH of 6.75), described by McKnight-Hanes and Whitford13 and modified by Amaechi et al.,2, at 37°C until the following erosive episode. Saliva was daily changed and the orange juice at each immersion.
Microhardness analysis along the erosive challenges
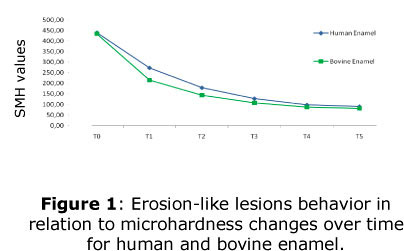
Surface microhardness measurements (SMH) were performed following the lesion progression at 250 um left from the measurements made at the post-indentation. SMH analyses were assessed under the same load and time described above for the baseline measurements. The analysis was carried out at the end of each day, after the two daily challenges, and to clarify this, six points in time were set (T0, T1, T2, T3, T4, T5) (Figure 1). The times are in equivalency with levels of the factor number of challenges, thus, T0 means the initial condition, while the time T5 refers to the end of the five day, which means, 10 erosive challenges.
Statistical Analysis
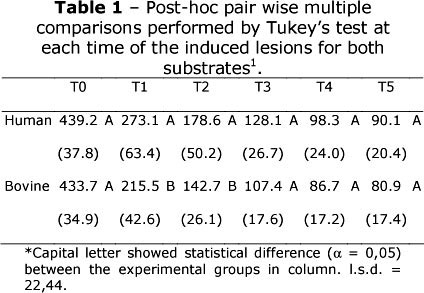
Data analysis was carried out using Statgraphics Centurion XV (Manugistics, Rockville, MD, USA) at a significance level of a = 0.05. After assumptions of normality and homogeneity of variances had been verified, SMH data were analyzed by two-way ANOVA as a split-plot design. Post-hoc pair wise multiple comparisons were performed using the Tukey's test applied at each time (T0, T1, T2, T3, T4 and T5) to identify differences in microhardness values between human and bovine substrate (L.S.D - 22.44). Regression analyses were used to model the SMH of human and bovine enamel, individually, over time.
RESULTS
The first null hypothesis was rejected, since human and bovine substrate showed differences regarding the SMH measurements. However, the second null hypothesis was accepted, regression analysis exhibits a quadratic polynomial reduction of SMH in function of the number of erosive challenges for both substrates. A two-way ANOVA according to a split-plot design revealed significant interaction between dental substrate and number of erosive challenges (p = 0.0096). At the times T0, T3, T4 and T5, SMH values of human and bovine enamel did not differ from each other, as shown in Table 1. After 2 and 4 erosive challenges (T1 and T2), human enamel showed higher SMH than the bovine substrate. The SMH values for both human and bovine enamel decreased SMH, according to the following models:
For human enamel:
SMH = 429,375-159,839*Time + 18,7089*Time2 (r2 = 90,3%)
For bovine enamel:
SMH = 409,545-175,832*Time + 22,6783*Time2 (r2 = 92,0%)
DISCUSSION
Erosive lesions involve enamel apatite crystals dissolution, which is associated with a surface softening caused by the frequent contact with acids. This process might weaken the enamel structure, and when the softened enamel is not re-hardened, they become more vulnerable to further dissolutions or physical insult, leading to an erosion lesion formation14,15,16.
Equivalency with levels of the factor number of challenges T0 = 0 pre challenge condition; T1 = after 2 challenges, or even, at the end of first day; T2 = after 4 challenges or even, at the end of second day; T3 = after 6 challenges, or even, at the end of third day; T4 = after 8 challenges or even, at the end of fourth day; T5 = after 10 challenges, or even, at the end of fifth day.
Orange juices may cause an appreciable level of erosion on enamel substrate2,6 and to simulate the acidic drinks intake, the model used to induce erosion like lesions was based on ready to drink orange juices. To induce erosion-like lesions, the chosen method was originally proposed for microradiographic assessments2, and as the study showed, the prolonged intermittent exposure of enamel slabs to orange juice increases progressively the depth of erosion lesion and the loss of mineral content, measured by microradiography2. However, although microradiography and even profilometry are accepted methods to assess erosion lesions11, it was not included in this study.
Since this study was designed to follow the initial stages of erosive lesions formation, preliminary tests showed that the protocol proposed by Amaechi et al., 2 was aggressive, thus, instead of six times a day for 24 days, the protocol was modified for twice daily challenges for five days. Regarding the response variable, as erosion has been considered a surface phenomenon17, to adapt the methodology, following the study objective, surface microhardness was chosen as evaluation method.
As a simple non-destructive method to assess the early stages of enamel erosion, indentation techniques have been extensively used to investigate enamel erosion by measuring the hardness of the enamel surface7,11,18. Surface Knoop microhardness was chosen, since this method allowed measurements along the time in the same specimen19, an aspect taken into account regarding the hypothesis initially proposed.
The SMH values throughout the elapsing process of lesions formation for both, human and bovine enamel showed a similar behavior (Figure 1). The adopted split plot design allow to obtain the equation of the fitted model for human and bovine enamel, and for both substrates the r2 observed has a value higher than 90%, which means that the data fitted tightly to the estimated model, and for both substrates, quadratic polynomial equations were obtained modeling the decrease of SMH values over time.
Considering that the microhardness of both substrates was assumed to be the same, previously to erosive challenge (T0) (Table 1), the SMH values of human and bovine enamel were similar; in fact, it has been showed that the mineral contents of both substrates are not significantly different1,5,8,10,20.
After the times T1 and T2 (Table 1), bovine enamel showed lower SMH values when compared to human substrate. Considering that bovine enamel had a faster erosive lesion progression than in human permanent enamel, our results corroborates with Amaechi et al.,2 and Attin et al.,5 , and, this finding may be related to the higher porosity of bovine enamel21. Microscopically, human and bovine enamel exhibit similar crystals orientations, although bovine teeth exhibit a wider inter-prismatic region9, with bigger crystals than human enamel,22 an structural aspect that may enhance the acid diffusion promoted by the erosive challenge acting on the tissue mechanical resistance, weakening the enamel structure, leading to lower microhardness values. Thus, the SMH values obtained in the times T1 and T2 of our study reinforces this suggestion, since microhardness indentations is a function of the mechanical properties of the material11.
Nevertheless, as the erosive process continues, in the times T3, T4 and T5, the difference between both substrates was no longer observed. The increase of erosive challenges along the time seems to be decisive to induce similar lesions in both human and bovine enamel. Despite the faster demineralization23 of bovine enamel due to its porosity21; with the high dissolution of apatite crystals caused by the acidic contact1, when hardness or even mineral content are reduced, the differences seems to be irrelevant. These results suggest that in the dependence of erosive challenges, the bovine enamel represents a viable alternative to human substrate.
CONCLUSION
Bovine substrate showed the most susceptible substrate to erosive challenge, but after several days of erosive challenge this difference disappears. On erosion research, the use of bovine tooth instead of human tooth seems to be a feasible alternative.
Acknowledgements
The authors are indebted to Patrícia Marchi for her technical assistance. This research was supported by CNPQ, process 111925/2004-2005 and his conduction was approved by the National Ethic Research Committee (CONEP), CAAE n° 0071.0.138.000-06.
REFERENCES
1. Meurman, R H, Frank, R M. Progression and surface ultrastructure of in vitro caused erosive lesions in human and bovine enamel. Caries Res 1991; 25(2): 81-87. [ Links ]
2. Amaechi BT, Higham SM, Edgar WM. Factors influencing the development of dental erosion in vitro: enamel type, temperature and exposure time. J Oral Rehabil 1999, 26: 624-30. [ Links ]
3. Ganss C, Klimek J, Schwarz N. A comparative profilometric in vitro study of the susceptibility of polished and natural human enamel and dentine surfaces to erosive demineralization Arch Oral Biol 2000; 45: 897-902. [ Links ]
4. Lussi A, Kohler N, Zero D, Schaffner M, Megert B. A comparison of the erosive potential of different beverages in primary and permanent teeth using an in vitro model. Eur J Oral Sci 2000; 108: 110-114. [ Links ]
5. Attin T, Wegehaupt F, Gries D, Wiegand A. The potential of deciduous and permanent bovine enamel as substitute for deciduous and permanent human enamel: Erosion-abrasion experiments. J Dent 2007; 35(10): 773-777. [ Links ]
6. Amaechi BT, Higham SM, Edgar WM. Techniques for the production of eroded lesions in vitro. J Oral Rehabil 1999b, 26: 97-102. [ Links ]
7. Attin T, Meyer K, Hellwig E, Buchalla W, Lennon AM. Effect of mineral supplements to citric acid on enamel erosion. Arch Oral Biol 2003; 48: 753-759. [ Links ]
8. Reeh ES, Douglas WH, Levine MJ. Lubrication of human and bovine enamel compared in an artificial mouth. Arch Oral Biol 1995; 40(11): 1063-1072. [ Links ]
9. Boyde A. Dental morphology and evolution. 1971, ed. Dahlberg A. A; Chicago University Press, Chicago, 81-94. [ Links ]
10. Mellberg JR: Hard-tissue substrates for evaluation of cariogenic and anti-cariogenic activity in situ. J Dent Res 1992 71 (special issue):913-19. [ Links ]
11. Barbour ME, Rees JS. The laboratory assessment of enamel erosion: a review. J Dent 2004; 32(8): 591602. [ Links ]
12. Fonseca RB, Haiter-Neto F, Fernandes-Neto AJ, Barbosa GAS, Soares CJ. Radiodensity of enamel and dentin of human, bovine and swine teeth. Arch Oral Biol. 2004; 49(11): 919-22. [ Links ]
13. Mcknight-Hanes C, Whitford GM. Fluoride release from three glass ionomer materials and the effects of varnishing with or without finishing. Caries Res 1992; 26: 345-50. [ Links ]
14. Imfeld T. Dental erosion. Definition, classification and links. Eur J Oral Sci 1996; 104: 151-155. [ Links ]
15. Zero DT. Etiology of dental erosion-extrinsic factors. Eur J Oral Sci 1996; 104: 162-77. [ Links ]
16. Barbour ME, Rees GD. The role of erosion, abrasion and attrition in tooth wear. J Clin Dent 2006; 17(4): 88-93. [ Links ]
17. Lussi A. Erosive tooth wear: a multifactorial condition of growing concern and increasing knowledge. Monograf Oral Sci 2006; 20:1-8. [ Links ]
18. Messias DC, Martins ME, Serra MC, Turssi CP. Feasibility of Using Sodium Bicarbonate Solution as a Damage-limiting Strategy for Erosion Lesions. Oral Health Prev Dent 2008; 6: 155-158. [ Links ]
19. Argenta RM, Tabchoury CP, Cury JA. A modified pH-cycling model to evaluate fluoride effect on enamel demineralization. Braz Oral Res. 2003 17(3): 241-6. [ Links ]
20. Edmunds DH, Whittaker DK, Green RM: Suitability of human, bovine, equine, and ovine tooth enamel for studies of artificial bacterial carious lesions. Caries Res 1988 22(6): 327-36. [ Links ]
21. Arends, J, Christoffersen, J, Ruben, J, Jongebloed, WL. Remineralization of bovine dentine in vitro. The influence of the F contents in solution on mineral distribution. Caries Res 1989; 23(5): 309-314. [ Links ]
22. Arends J, Jongebloed WL. Crystallites dimensions of enamel. J. Biol Bucca, 1978; 6:161-71. [ Links ]
23. Featherstone, JDB, Mellberg, JR. Relative rates of progress of artificial carious lesions in bovine, ovine, and human enamel. Caries Res 1981; 15(1); 109114. [ Links ]
 Correspondence:
Correspondence:
Francisco Carlos Rehder Neto
Departamento de Odontologia Restauradora
Faculdade de Odontologia de Ribeirão Preto - USP
Av. do Café, s/n Monte Alegre
Cep: 14040-904 Ribeirão Preto - SP, Brazil
e-mail: fcrehder@usp.br
phone: 55-16-3602-4075
fax: 55-16-3633-4187
Recebido em 07/01/2010
Aprovado em 18/03/2010













