Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.60 no.3 Porto Alegre Jul./Set. 2012
ORIGINAL / ORIGINAL
In vitro antibacterial and non-stick activity of extracts from leaves of Psidium guineense Sw. and Syzygium cumini (L.) Skeels on oral microorganisms
Atividade antibacteriana e antiaderente in vitro dos extratos das folhas de Psidium guineense Sw. e Syzygium cumini (L.) Skeels sobre microrganismos orais
Thiago Isidro VIEIRA I; Brenna Louise Cavalcanti GONDIM I; Bianca Marques SANTIAGO II; Ana Maria Gondim VALENÇA II
I Universidade Federal da Paraíba, Faculdade de Odontologia. Cidade Universitária, 58051-900, João Pessoa, PB, Brasil
II Universidade Federal da Paraíba, Faculdade de Odontologia, Departamento de Clínica e Odontologia Social. João Pessoa, PB, Brasil
ABSTRACT
Objective
This study determined the Minimum Inhibitory Concentration and the Minimum Inhibitory Concentration of Adherence of hydroalcoholic extracts of the leaves of strawberry guava (Psidium guineense Sw.) and of the jambolan (Syzygium cumini (L.) Skeels) against Streptococcus mutans (ATCC 25175), Streptococcus oralis (ATCC 10557), Streptococcus parasanguis (ATCC 903), Streptococcus salivarius (ATCC 7073), Streptococcus sp (ATCC 15300), and Lactobacillus casei (ATCC 9595).
Methods
Strains were seeded on blood agar plates to determine the Minimum Inhibitory Concentration by the agar-diffusion technique. The inclined tubes technique was used for Minimum Inhibitory Concentration of Adherence evaluation in the presence of 5% of sucrose, in Mueller-Hinton broth. The same procedures were accomplished with the 0.12% chlorhexidine digluconate (positive control). Assays were performed in duplicate. The plates and the tubes were maintained in microaerophillia at 37ºC for 24 hours.
Results
The Minimum Inhibitory Concentration obtained for strawberry guava extract ranged from 275 to 1100 mg.ml-1. Jambolan values were 242.5 to 485 mg.mL-1; and 0.12% chlorhexidine digluconate were 75x10-3 to 9x10-3 mg.ml-1. The Minimum Inhibitory Concentration of Adherence reported the following values : strawberry guava (1.81 to 28.94 mg.ml-1); jambolan (1.60 to 12.76 mg.ml-1) and 0.12% chlorhexidine didigluconate (4.93 x10-4 to 19.70 x10-4 mg.ml-1).
Conclusion
It was concluded that the hydroalcoholic extracts from the leaves of P. guineense Sw. and S. cumini (L.) Skeels presented antimicrobial and nonstick effect on the tested lineages; further studies are needed to confirm these extracts to be natural antibacterial agents for use in controlling dental caries.
Indexing terms: Anti-bacterial agents. Biofilms. Phytotherapy. Psidium. Syzygium jambolanum.
RESUMO
Objetivo
Determinar a Concentração Inibitória Mínima e a Concentração Inibitória Mínima de Aderência dos extratos hidroalcoólicos das folhas do araçá (Psidium guineense Sw.) e do jambolão (Syzygium cumini (L.) Skeels) frente à Streptococcus mutans (ATCC 25175), Streptococcus oralis (ATCC 10557), Streptococcus parasanguis (ATCC 903), Streptococcus salivarius (ATCC 7073), Streptococcus sp (ATCC 15300) e Lactobacillus casei (ATCC 9595).
Métodos
As cepas foram semeadas em placas de ágar sangue para determinação da CIM pela técnica de ágar-difusão. Utilizou-se a técnica dos tubos inclinados para avaliação da Concentração Inibitória Mínima de Aderência ao vidro, na presença de 5% de sacarose, em caldo Mueller-Hinton. Os mesmos procedimentos foram realizados com o digluconato de clorexidina à 0,12% (controle positivo). Os ensaios foram realizados em duplicata. As placas e os tubos foram mantidos em microaerofilia a 37ºC por 24 horas. Os dados foram analisados descritivamente.
Resultados
As Concentrações Inibitórias Mínimas obtidas para o extrato do araçá variaram de 275 a 1100 mg.ml-1. Para o extrato do jambolão apresentaram valores de 242,5 a 485 mg.ml-1. E quanto ao digluconato de clorexidina à 0,12% foram de 9x10-3 a 75x10-3 mg.ml-1. Quanto às Concentrações Inibitórias Mínimas de Aderência, registrou-se os seguintes valores: araçá (1,81 a 28,94 mg.ml-1); jambolão (1,60 a 12,76 mg.ml-1) e digluconato de clorexidina à 0,12% (4,93x10-4 a 19,70x10-4 mg.ml-1).
Conclusão
Conclui-se que os extratos hidroalcoólicos das folhas de P. guineense Sw. e S. cumini (L.) Skeels apresentaram efeito antimicrobiano e antiaderente sobre as linhagens testadas, sendo necessários estudos complementares que confirmem ser estes extratos alternativas de antibacterianos naturais no controle da cárie dentária.
Termos de indexação: Antibacterianos. Biofilmes. Fitoterapia. Psidium. Syzygium jambolan.
INTRODUCTION
Dental caries is an infectious disease that is disseminated all around the world, and according to the SB Brazil Project 2002/20031, it is one of the most frequent oral disorders with a prevalence of 40.62% at the age of twelve.
Dental biofilm is the main etiological factor of caries and it is characterized as a community of microorganisms that adheres to the tooth surface, with S. mitis and S. sanguis being the pioneer bacteria. The presence of S. mutans and S. Sobrinus is most prevalent in the initial stages of caries. The L. casei is found during the development of cavities2. S. oralis and S. salivarius are also present in the biofilm. To explain multifactorial nature of caries, not only the biological factors should be included (biofilm, saliva and diet), but the modulating factors (income, education, behavioral factors, among others), which indirectly influence, to a greater or lesser extent, the probability of an individual developing this disease, must also be taken into consideration. However, the associations found between life and health conditions play an important role in the health-disease process and in places with a low social, cultural and economic level, this pathology is frequently more prevalent and severe3.
Diseases, among them caries, are related to the lack of basic sanitation in developing countries, in addition to malnutrition and the difficulty of access to medication. Within this context, phytotherapy is widely practiced, and the popular use of traditionally established medicinal plants has been taken as a guide for different studies4. In a study conducted in the city of Cedro (Ceara, Brazil), it was observed that 82.5% of the population used phytotherapy and 49.5% used it to treat oral diseases5.
Several medicinal plants have been used for prophylactic and curative purposes and those described in the present study are among them. Several studies have been conducted to find new plants with anti-microbial activity6.
Psidium species belongs to the Myrtaceae family and it is native to tropical America. Traditionally, plants of the Psidium spp. Species are used to treat scurvy in Asia and Africa; cough and lung dysfunctions in Bolivia and Egypt; as an anti-inflammatory and hemostatic agent in China, and as an anti-diarrhea compound in Mexico7,8. However, the antibacterial activity against oral pathogens has not yet been studied.
The compounds with known antibacterial activity and present in P. guineense Swartz are flavonoids (phenolic compounds such as avicularin, guaijaverin, tannins and quercetin). Tannins are very reactive chemically and form intra- and inter-molecular hydrogen bonds, are easily oxidized both through specific plant enzymes and under the influence of metals. Both their ecological properties for insect, fungal, and bacterial control and their pharmacological activities are based on their ability to form complexes with macromolecules such as proteins10.
The astringency of the jambolan pulp is due to the presence of tannins, high molecular weight phenolic compounds, which are also present in fruit such as cashew (Anacardium spp.) and green banana (Musa sp.). As the fruit ripens, there is commonly a reduction in astringency, and this is attributed to the loss of tannin solubility. However, in small proportions or in combination with other food components, the astringency may contribute to a desirable flavor, such as wines made from pigmented grape cultivars. When ingested in large amounts, tannins may precipitate proteins, inhibit digestive enzymes and affect the absorption of vitamins and minerals; thus, they may also be considered nutritionally undesirable. However, at present, the negative results involving this class of phenolic compounds in the diet have been reviewed11.
The plants with therapeutic properties used in traditional health care are an important source of new biologically active compounds12. The interest in medicinal plants is now directed to the production of therapeutic formulae with low toxicity and cost. This is significantly important for countries with poor financial resources, but rich in biodiversity13.
In view of the above considerations, it is believed that it may be possible to use hydroalcoholic extracts of strawberry guava and jambolan as auxiliaries in the treatment and control of dental caries, since the phytotherapeutic compounds have shown satisfactory results, in addition to being low cost, which would make it possible for them to be used by population groups with limited access to health care.
Following this reasoning, the present study assessed the antimicrobial activity and non-stick effect of the hydroalcoholic extract of the leaves of P. guineense Sw. (strawberry guava) and S. cumini (L.) Skeels (jambolan) against bacteria present in the dental biofilm.
METHODS
Obtaining vegetal material and taxonomic identification
The raw material (healthy leaves) of P. guineense Sw. and S. cumini (L.) Skeels were obtained at campus I of the Federal University of Paraiba (UFPB) in January and February of 2010 and botanically identified and registered by the herbalist Professor Lauro Pires Xavier (JPB), Custodian of National Genetic Heritage. (Psidium guineense Sw.: Material examined: Brazil, Paraíba: João Pessoa, Campus I of UFPB), 7°06'54'' S, 34°51'47'' W, Alt: 47m, May 14, 2010, fl., T.I. Vieira 01 (Voucher Number JPB 44354); Syzygium cumini (L.) Skeels: Material examined: Brazil, (xxx), 7°06'54'' S, 34°51'47'' W, Alt: 47m, May 14, 2010, fr., T.I. Vieira 02 (Voucher Number JPB 44355).
Preparation of phytotherapic extracts
The hydroalcoholic extraction was performed from the previously selected (775g of each plant material) and botanically identified strawberry guava and jambolan leaves (70% ethanol and distilled water in the ratio 7:3) for a period of 7 consecutive days. After this extraction time interval (by the maceration method), the product was denominated raw extract. The extract obtained was concentrated at a temperature of 55 to 60°C in an oven (Fabbe Primar Industrial Ltda, Sao Paulo, Brazil) for complete removal of water for 48 hours. The material obtained after concentration was in the form of a paste with concentrations of 1.1 g ml-1 and 0.97 g ml-1 of strawberry guava and jambolan, respectively.
Determining the minimum inhibitory concentration
The hydroalcoholic extracts of P. guineense Sw and S. cumini (L.) Skeels and 0.12% chlorhexidine digluconate solution (Periogard®, Colgate, São Paulo, Brazil) diluted in sterile distilled water were tested at concentrations between 1100 to 0.27 mg ml-1, 970 to 0.47 mg ml-1 and 1.2 to 0.0006 mg ml-1, respectively. Standard strains of S. mutans (ATCC 25175), S. oralis (ATCC 10557), S. parasanguis (ATCC 903), S. salivarius (ATCC 7073), S. sp (ATCC 15300) and L. casei (ATCC 9595) were used from the National Institute of Quality Control in Health, Oswaldo Cruz Foundation. The strains were reactivated in BHI broth (DIFCO®, São Paulo, Brazil) in a bacteriological oven (Quimis Aparelhos Científicos Ltda., São Paulo, Brazil) in microaerophilia.
Once the bacteria were reactivated, the minimum inhibitory concentration in blood agar was determined by the agar diffusion technique and the inoculum (106 CFU / ml) was seeded on Petri dishes (90mm/15mm, Alfa-lab®, Sao Paulo, Brazil) containing 20 ml of solid medium (BHI agar plus 5% defibrinated sheep blood) with the aid of swabs with subsequent well drilling of approximately 6 mm in the solid medium to place 50μL of the dilutions. Next, the plates were incubated in microaerophilia at 37ºC for 24 hours. All the tests were performed in duplicate. The minimum inhibitory concentration was defined as the lowest concentration of the extract capable of producing an inhibition zone of bacterial growth.
Determining the minimum inhibitory concentration of adherence
The minimum inhibitory concentration of adherence of the bacteria on the glass was determined in the presence of 5% sucrose (INLAB®, Diadema, Brazil) in the Mueller-Hinton broth (DIFCO®, São Paulo, Brazil) using concentrations of the hydroalcoholic extracts of strawberry guava (28.94 to 0.007 mg ml-1) and jambolan (25.52 to 0.012 mg ml-1) and the 0.12% chlorhexidine digluconate solution (0.032 to 1.5 x 10-5 mg ml-1) by means of the inclined tube technique at 30°. From the growth, the strains were sub-cultivated at 37ºC in BHI broth in microaerophilia for 24 hours. The minimum inhibitory concentration of adherence was defined as the lowest concentration of the extract in the 5% sucrose medium that prevented bacterial adhesion to the glass tube.
Data Analysis
The results related to the antimicrobial and non-stick activity of the extracts studied and 0.12% chlorhexidine digluconate were assessed by means of descriptive statistics to obtain the mean inhibitory halos and determine the minimum inhibitory concentration of adherence. Inhibition halos of bacterial growth equal to or greater than 7mm were recorded as inhibitory activity14.
RESULTS
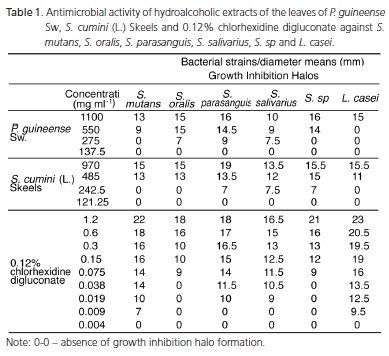
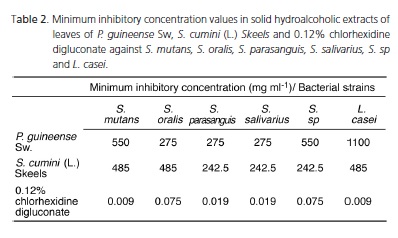
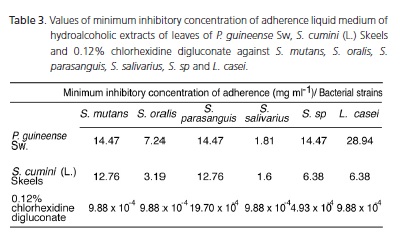
In Table 1, the data with reference to the antimicrobial activity are shown. In Table 2, the values of minimum inhibitory concentrations obtained from the P. Guineense Sw extract ranged from 275 to 1100 mg.ml-1. The values of the S. cumini (L.) Skeels extract ranged from 242.5 to 485 mg.ml-1. The values for 0.12% chlorhexidine digluconate ranged from 9x10-3 to 75x10-3 mg.ml-1. With respect to the minimum inhibitory concentrations of adherence, the following values were recorded: strawberry guava (1.81 to 28.94 mg.ml-1); jambolan (1.60 to 12.76 mg.ml-1) and 0.12% chlorhexidine digluconate (4.93x10-4 to 19.70x10-4 mg.ml-1), according to Table 3.
DISCUSSION
The results demonstrated the effectiveness of the mentioned extracts against the microorganisms tested. All strains were susceptible to the extracts with growth inhibition halos that ranged from 7 to 19mm. There was a proportional reduction in the diameter of the halos, as the concentration of the extract was reduced, as shown in Table 1.
Growth inhibition was shown to be homogeneous with regard to 0.12% chlorhexidine digluconate, according to its concentration. A similarity in proportion was found between the reduction in diameter of the inhibition halos and reduction in the concentration of the substance. The inhibition halos ranged from 7 to 23 mm, as shown in Table 1.
The results of laboratory studies may not reflect the real effect of a material when applied in an in vivo condition15. Therefore, the results obtained cannot be extrapolated to a clinical situation. However, in vitro research gives support to clinical trials.
It is difficult to compare results obtained in this study with some reported in the literature since the composition of the hydroalcoholic extracts may vary even within the same species due to different harvesting periods, different extraction methods, different microbiological tests used, and sensitivity of the strains16.
Although the concentrations of the components of the extracts of the strawberry guava and jambolan leaves were not evaluated in the present study, it was noted that some studies have analyzed the composition of the fruit of these species and found that the components found in P. guineense Sw. were phenolic compounds of the ellagitannin and gallotannin type. As regards S. cumini (L.) Skeels structures of gallotannins, ellagitannins, flavonols and flavonoids were observed. Several components derived from myricetin were also identified in the fruit Syzygium. Other elements detected in the fruit were also present in the leaf, such as myricetin acylated deoxyhexose17.
Chromatography consists of a physico-chemical method of separation based on differential migration of the components of a mixture being used to identify compounds by analogy to pre-existing standards for purification of compounds, among other purposes. In the present study, chromatographic evaluation of hydroalcoholic extracts of P. guineense Sw. and S. cumini (L.) Skeels was not performed, so that it was not possible to compare the chromatographic profiles. Nevertheless, from the antibacterial tests against Gram-positive bacteria, it was found that the behavior of these extracts was similar to that observed by other researchers, thus allowing comparisons to be made18.
The present test, by means of the agar diffusion technique, demonstrated the antimicrobial action of hydroalcoholic extracts of strawberry guava and jambolan leaves against biofilm-forming strains. The agar diffusion technique is used as a standard test to measure the antibacterial properties of materials, in spite of its limitations; one of these being that it only measures components that are soluble in water19.
The use of the leaves for the preparation of hydroalcoholic extracts was chosen, because they are easily collected and available. Furthermore, the leaf is the main raw material used by Brazilian pharmaceutical laboratories to manufacture phytomedicines20.
In the present study, the aim was to investigate the action of hydroalcoholic extracts of P. guineense Sw. e S. cumini (L.) Skeels against the strains of S. mutans, S. oralis, S. parasanguis, S. salivarius, S. sp and L. casei. These plant species were chosen because their activity against oral pathogens has not been studied9.
The antimicrobial activity presented by the plants in the family Myrtaceae is related to its high content of essential oils and phenolic compounds, such as tannins21.
When conducting a phytochemical study and study on antimicrobial activity of P. guineense Sw. against S. mutans, González et al.22 observed that the antibacterial effect could be attributed to secondary metabolites such as tannins, flavonoids, terpenes, aldehydes, among others, which were present in the fruit of the species. Similar to those reported in the present study, the inhibition halos recorded ranged from 15 mm to 50 mm. It is important to point out that González et al.22 used ethanolic extracts while the present research used hydroalcoholic extracts of the leaf.
Brighenti et al.9 conducted a research about the feasibility, protein expression and acid production of the strains of S. mutans under the effect of the aqueous extract obtained by decoction in distilled water of the Psidium cattleianum leaf (red strawberry guava) and found that the combination of tannins and flavonoids present in this vegetable acted as an inhibitory agent and inhibitor of the expression and activity of certain enzymes. Gaetti- Jardim Júnior et al.23 investigated the antibacterial action of the hydroalcoholic extract of the bark and leaf of red guava against S. mutans ATCC 35668 and found that the extract showed effect even with the dilution ratio of 1:68. Although the species studied were common guava, sour guava or field guava, the combination found in the red strawberry guava might indicate how the inhibitory effect observed in the common guava works since both belong to the same Myrtaceae family.
The ethanolic extract of S. cumini (L.) Skeels showed one of the highest antimicrobial activities, inhibiting 83.3% of the bacteria resistant to antibiotics14. In this study, the Minimum Inhibitory Concentration of jambolan was 50, 300 and 400 mg/mL against Pseudomonas aeruginosa, Staphylococcus aureus and Enterobacter aerogenes, respectively. Similar concentrations were also found in the present study as shown in Table 2. However, it should be noted that these concentrations were found for noncariogenic bacteria, differing from the strains used here. In a previous study, the jambolan extract inhibited 40% of the microorganisms and it was observed that this effect was evident also against bacteria resistant to antibiotics, which were isolated in a hospital environment6.
Loguércio et al.21 assessed antibacterial activity of the hydroalcoholic extract of jambolan leaves by the disk agar diffusion method. The authors found antimicrobial effect on 17 bacterial isolates, both gram-positive and gram-negative bacteria, but not on oral streptococci, which was the aim of their test. Another difference was the agar diffusion technique used. The agar well diffusion method was adopted. The activity of jambolan is due to the presence of tannins (phenolic polymeric substances) that are able to form complexes with proteins, among other functions.
Similarly, other studies investigated the antimicrobial potential of jambolan extracts against gram-positive bacteria and found that the methanol extract of S. cumini (L.) Skeels inhibited the growth of Bacillus subtilis ATCC 6633 and Staphylococcus aureus ATCC 2973724. Although the present study did not assess antifungal activity, however, some trials analyzed the capacity of jambolan to inhibit the fungal development25-26. Höfling et al.25 investigated the antifungal activity of methanolic and dichloromethanolic extracts against various Candida strains and found that the strains were all sensitive to concentrations ranging from 0.001 to 0.03 mg/mL. Jabeen et al.26 assessed the capacity of the jambolan extract to inhibit Ascochyta rabiei and found that the aqueous extract of the leaves showed significant suppression of up to 30% of fungal growth.
Voss-Rech et al.27 analyzed the capacity of several hydroethanolic extracts, among them, of the jambolan extract to inhibit the growth of different species of Salmonella. The minimum inhibitory concentrations found ranged from 40 to 320 mg/mL, similar to the data shown in Table 2, although oral microorganisms were used.
Plants subjected to the tests of minimum inhibitory concentration of adherence showed non-stick activity, as shown in Table 3. This mechanism of adhesion of bacterial cells to tooth surfaces can be explained by the presence of cell surface adhesins that bind to specific sites on the host as some receptors present on the film acquired28. Similarly, Gibbons28 reports that some factors may affect bacterial adherence such as the influence of salivary secretions, influence of lectins from diet, and the influence of certain antibiotics. It is suggested that the components present in the strawberry guava and jambolan have a non-stick action against the strains tested.
There are no studies in the pertinent literature that investigate the non-stick effect of these species on oral microorganisms. Thus, interference in the adhesion mechanism may be a strategy to control biofilm, thus preventing oral diseases such as dental caries and periodontal diseases.
In the present trial, the extracts mentioned were effective in inhibiting adhesion to glass; however, these concentrations were higher than those of 0.12% chlorhexidine digluconate, as shown in Table 2. Chlorhexidine digluconate is considered a biocompatible disinfectant able to reduce the microbial population and its significant substantivity29. It has been widely used to control dental biofilm formation, altering the composition of supragingival biofilm.
With regard to cytotoxicity, a previous study investigated this effect on the leaves of S. cumini (L.) Skeels and it was found that jambolan showed no cytotoxicity or mutagenicity in the system of the test animal (in bone marrow cells of Wistar rats) and plant (in meristematic cells of Allium cepa L.)30. Although the results of this in vitro study support the use of extracts of these plants, popular use of these plants to control dental caries is not recommended without conducting further studies on toxicity, cytotoxicity, mutagenicity, among others.
Further tests using bacteria on individual and associated biofilm should be conducted to confirm the antimicrobial potential of the extracts of P. guineense Sw. and S. cumini (L.) Skeels, as well as to determine the minimum bactericidal concentration, thus enabling a better understanding about the action of these extracts and a more accurate analysis of the results that will be obtained.
CONCLUSION
It may be concluded that the hydroalcoholic extracts of the leaves of P. guineense Sw. and S. cumini (L.) Skeels showed a nonstick and antimicrobial effect on the strains tested, but further studies are necessary to confirm whether these natural antibacterial alternatives are effective in the control of dental caries.
Collaborators
TI VIEIRA prepared the research project, conducted microbiological tests and wrote the article. BLC GONDIM participated in the microbiological test and writing of the article. BM SANTIAGO and AMG VALENÇA supervised the research and participated in the writing of the article.
REFERENCES
1. Brasil. Ministério da Saúde. Projeto SB Brasil 2003: condições de saúde bucal da população brasileira 2002-2003. Resultados principais. Brasília: Ministério da Saúde; 2004.
2. Nyvad B, Marsh PD. A microbiota oral e biofilmes formados sobre os dentes. In: Fejerskov O, Kidd E. Cárie dentária: a doença e seu tratamento clínico. São Paulo: Santos; 2007. p. 29-48.
3. Pereira AC. Tratado de saúde coletiva em odontologia. Nova Odessa: Napoleão; 2009.
4. Kumate J. Infectious disease in the 21st century. Arch Med Res. 1997;28(2):155-61.
5. Lima Júnior JF, Vieira LB, Leite MJVF, Lima KC. O uso de fitoterápicos e a saúde bucal. Saúde Rev. 2005;7(16):11-7.
6. Michelin DC, Moreschi PE, Lima AC, Nascimento GGF, Paganelli MO, Chaud MV. Avaliação da atividade antimicrobiana de extratos vegetais. Rev Bras Farmacog. 2005;15(4):316-20.
7. Lozoya X, Meckes M, Abou-Zaid M, Tortoriello J, Nozzolillo C, Amason JT. Quercetin glycosides in Psidium guajava L. leaves and determination of spasmolytic principle. Arch Med Res. 1994;25(1):11-5.
8. Jaiarj P, Khoohaswan P, Wongkrajang Y, Peungvicha P, Suriyawong P, Saraya ML, et al. Anticough and antimicrobial activities of Psidium guajava Linn leaf extract. J Ethnopharmacol. 1999;67(2):203-12.
9. Brighenti FL, Luppens SBI, Delbem ACB, Deng DM, Hoogenkamp MA, Gaetti-Jardim JR E, et al. Effect of Psidium cattleianum leaf extract on Streptococcus mutans viability, protein expression and acid production. Caries Res. 2008;42(2):148-54. doi: 10.1159/000121439.
10. Santos SC, Mello JCP. Taninos. In: Simões CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR. Farmacognosia: da planta ao medicamento. Porto Alegre: UFRGS; 1999. p. 614- 56.
11. Agostini-Costa TS, Silva DB. Jambolão: a cor da saúde. 2008 [citado 2009 Mar 22]. Disponível em: <http://www.infobibos. com/Artigos/2008_1/Jambolao/index.htm>.
12. Lima IO, Oliveira RAGO, Lima EO, Farias NMP, Evandro Leite de Souza EL. Atividade antifúngica de óleos essenciais sobre espécies de Candida. Rev Bras Farmacog. 2006;16(2):197-201. doi: 10.1590/S0102-695X2006000200011.
13. Gossell-Williams M, Simon OR, West ME. The past and present use of plants for medicines: a review. West Indian Med J. 2006;55(4):217-8.
14. Nascimento GGF, Locatelli J, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibioticresistant bacteria. Braz J Microbiol. 2000;31(4):247-56. doi: 10.1590/S1517-83822000000400003.
15. Souza-Gugelmin MCM, Silva FWGP, Queiroz AM, Amaral THA. Avaliação da atividade antimicrobiana de dentifrícios infantis: estudo in vitro. Rev Fac Odontol Porto Alegre. 2006;47(3):10-3.
16. Tampieri MP, Galuppi R, Macchioni F, Carelle MS, Falcioni L, Cioni PL, et al. The inhibition of Candida albicans by selected essential oils and their major components. Mycopathologia 2005;159(3):339-45. doi: 10.1007/s11046-003-4790-5.
17. Gordon A, Jungfer E, Silva BA, Maia JG, Marx F. Phenolic constituents and antioxidant capacity of four underutilized fruits from the Amazon region. J Agric Food Chem. 2011;59(14):7688- 99. doi: 10.1021/jf201039r.
18. Buriol L, Finger D, Schmidt ED, Santos JMT, Rosa MR, Quináia SP, et al. Composição química e atividade biológica de extrato oleoso de própolis: uma alternativa ao extrato etanólico. Quim Nova. 2009;32(2):296-302. doi: 10.1590/S0100- 40422009000200006.
19. Tobias RS. Antibacterial properties of dental restorative materials: a review. Int Endod J. 1988;21(2):155-60. doi: 10.1111/j.1365- 2591.1988.tb00969.x.
20. Di Stasi LC, Oliveira GP, Carvalahes MA, Queiroz-Junior M, Tien OS, Kakinami SH, et al. Medicinal plants popularly used in the Brazilian Tropical Atlantic Forest. Fitoterapia. 2002;73(1):69-91. doi: /10.1016/S0367-326X(01)00362-8.
21. Loguércio AP, Battistin A, Vargas AC, Henzel A, Witt NM. Atividade antibacteriana de extrato hidro-alcoólico de folhas de jambolão Syzygium cumini L. Skells. Ciên Rural. 2005;35(2):371-6.
22. Neira Gonzalez AM, Ramirez Gonzalez MB, Sanchez Pinto NL. Estudio fitoquímico y actividad antibacterial de Psidium guineense Sw (choba) frente a Streptococcus mutans, agente causal de caries dentales. Rev Cubana Plant Med. 2005;10(3- 4):3-4.
23. Gaetti-Jardim Júnior E, Landucci LF, Gaetti-Jardim EC, Sangalli J, Sousa FRN. Atividade inibitória de extratos do cerrado brasileiro sobre microrganismos anaeróbios e associados a infecções nosocomiais. Rev Bras Cienc Saúde. 2009;13(2):43-52.
24. Kaneria M, Baravalia Y, Vaghasiya Y, Chanda S. Determination of antibacterial and antioxidant potential of some medicinal plants from Saurashtra Region, India. Indian J Pharm Sci. 2009;71(4):406-12. doi: 10.4103/0250-474X.57289.
25. Höfling JF, Anibal PC, Obando-Pereda GA, Peixoto IAT, Furletti VF, Foglio MA, et al. Antimicrobial potential of some plant extracts against Candida species. Braz J Biol. 2010;70(4):1065- 8. doi: 10.1590/S1519-69842010000500022.
26. Jabeen K, Javaid A. Antifungal activity of Syzygium cumini against Ascochyta rabiei-the cause of chickpea blight. Nat Prod Res. 2010;24(12):1158-67. doi: 10.1080/14786410902941154.
27. Voss-Rech D, Klein CS, Techio VH, Scheuermann GN, Rech G, Fiorentin L. Antibacterial activity of vegetal extracts against serovars of Salmonella. Cienc Rural. 2011;41(2):314-20. doi: 10.1590/S0103-84782011000200022.
28. Gibbons RJ. Microbial ecology adherent interactions which may affect microbial ecology in the mouth. Journ Dent Res. 1984; 63(3): 378-85.
29. Ferraz CCR, Gomes BPFA, Zaia AA, Teixeira FB, Souza-Filho SJ. In vitro assesment of antimicrobial action and mechanical ability of chlorexidine gel as an endodontic irrigant. J Endod. 2001;27(7):452-55. doi: 10.1177/00220345840630030401.
30. Vicentini VEP, Camparoto ML, Teixeira RO, Mantovani MS. Averrhoa carambola L., Syzygium cumini (L.) Skeels and Cissus sicyoides L.: medicinal herbal tea effects on vegetal and animal test systems. Acta Scientiarum. 2001;23(2):593-8.
 Correspondence to:
Correspondence to:
TI VIEIRA
e-mail: thiago_isidro@yahoo.com.br
Received on: 29/9/2011
Final version resubmitted on: 4/4/2012
Approved on: 16/5/2012













