Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RGO.Revista Gaúcha de Odontologia (Online)
versão On-line ISSN 1981-8637
RGO, Rev. gaúch. odontol. (Online) vol.61 no.2 Porto Alegre Abr./Jun. 2013
ORIGINAL / ORIGINAL
Detection of Streptococcus mutans of the spaP gene and dental caries in mother/child pairs
Detecção de Streptococcus mutans portador do gene spaP e cárie dentária em pares mãe/criança
Flávio José Sambatti PIERALISEI; Sandra Mara MACIELI; Flaviana Bombarda de ANDRADEII; José Eduardo GARCIAIII; Regina Célia POLI-FREDERICOI
I Universidade Norte do Paraná, Faculdade de Odontologia. Av. Paris, 675, 86041-120, Londrina, PR, Brasil.
II Universidade de São Paulo, Faculdade de Odontologia, Departamento de Endodontia e Materiais Dentários. Bauru, SP, Brasil.
III Universidade Federal de Pernambuco, Centro de Ciências Biológicas, Departamento de Zoologia, Laboratório de Genômica Evolutiva. Recife, PE, Brasil.
ABSTRACT
Objective
Objective: To detect the presence and transmission of S. mutans carrier of the spaP gene in samples of bacterial plaque in mother/child pairs from municipal child education centers, and the possible association with dental caries.
Methods
The sample comprised 56 mother/child pairs. For the evaluation of the prevalence and severity of caries, the DMFT and dmft indices were used, following World Health Organization criteria. The oral hygiene pattern was also evaluated using the Simplified Oral Hygiene Index and O'Leary's plaque control index. Using DNA extracted from bacteria in the dental plaque of mother/child pairs, a sequence of the S. mutans spaP gene was amplified using PCR. The chi-squared test, Fisher's exact test and Pearson's correlation coefficient were applied, using a level of significance of p< 0.05.
Results
The mothers presented a DMFT index of 11.02 (SD=6.3) while the children exhibited a dmft index of 2.09 (SD=3.2). Approximately 40% of mother/child pairs had a good oral hygiene index. A higher percentage of children with caries had, in their dental plaque, S. mutans harboring the spaP+ gene (p=0.03). No association was detected between the presence of S. mutans spaP+ in the dental plaque of mother and child.
Conclusion
An association was found between experience of caries in the children and the presence of bacteria carrying the spaP+ gene. Our results did not detect vertical transmission.
Indexing terms: Dental caries. Polymerase chain reaction. Streptococcus mutans. Transmission.
RESUMO
Objetivo
Detectar a presença e a transmissão de S. mutans portador do gene spaP em amostras de placa bacteriana em pares mãe/criança de centros municipais de educação infantil e possível associação com a cárie dentária.
Métodos
A amostra foi composta por 56 pares mãe/criança. Para avaliação da prevalência e severidade de cárie foram utilizados os índices CPO-D e ceo-d seguindo critérios da Organização Mundial de Saúde. O padrão de higiene bucal foi avaliado através do Índice de Higiene Oral Simplificado e do índice de controle de placa O´Leary. Utilizando o DNA extraído das bactérias da placa dentária dos pares mãe/criança, uma seqüência do gene spaP de S. mutans foi amplificado pela PCR. Os testes de Qui-quadrado, exato de Fisher e correlação de Pearson foram realizados. Adotando-se significância de p< 0,05.
Resultados
As mães apresentaram índice CPO-D de 11,02 (DP=6,3) enquanto as crianças mostraram índice ceo-d de 2,09 (DP=3,2). Aproximadamente 40% dos pares mãe/criança tinham um bom índice de higiene bucal. Maior porcentagem das crianças com cárie apresentaram em sua placa dentária S. mutans abrigando o gene spaP+ (p=0,03). Não foi detectada associação entre a presença de S. mutans spaP+ na placa dentária da mãe e seu filho.
Conclusão
Foi verificada a associação entre a experiência de cárie nas crianças e a presença da bactéria portadora do gene spaP+. Nossos resultados não detectaram a transmissão vertical.
Termos de indexação: Cárie dentária. Reação em cadeia da polimerase. Streptococcus mutans. Transmissão.
INTRODUCTION
A large part of the world's population is affected by dental caries. Despite the multiple factors associated with the occurrence of dental caries in the various population sub-groups, there is evidence that it may be a controllable disease1.
Streptococcus mutans is a good indicator of caries; in general, the greater the amount of S. mutans in the oral cavity, the greater the chance of developing this disease. However, its presence in high numbers only shows that the oral environment is appropriate for the onset or progression of dental caries2.
These bacteria may be acquired through both vertical and horizontal transmission, the mothers being the main source of infection through S. mutans in their children3. Other studies present the detection of different genotypes in children which were not found in the respective mothers or other family members, suggesting alternative paths for S. mutans transmission, such as horizontal transmission4-9.
Streptococcus mutans are frequently isolated from dental plaque and carious lesions. Various methods have been used to identify these bacteria including biochemical tests10, serological tests11, DNA probes12 and through modern techniques in molecular biology such as via polymerase chain reaction (PCR) of streptococci in the mutans group in the saliva or in the dental plaque13. Of these, the PCR method, being quick, sensitive and simple, has been used to detect potential oral pathogens and identify cariogenic bacteria. The target genes for the PCR may be related to virulence factors such as the dextranase gene (dex)14 or the spaP gene15.
The implantation of S. mutans in the oral cavity is promoted by the presence of bacterial adhesins that interact with the saliva receptors. During the initial adherence of the S. mutans, it is necessary for it to produce the protein called 190 kDa fibrillar adhesin, known as antigen I/II (Ag I/II)15. The gene which codifies this adhesion in S. mutans was cloned by Lee et al.16 and called spaP, and then later on by Okahashi et al.17, who named it pac.
Based on the fact that S .mutans adheres to the tooth enamel through the Ag I/II and that this constitutes a genetic marker for potentially cariogenic lineages, Ono et al.15 implemented a system to amplify the DNA fragment related to this antigen, in the region comprising nucleotides 3668 to 3859 of the spaP gene, using PCR in microbiological isolates from the dental plaque. It should be stressed that this region of the spaP gene does not show any similarity to other sequences of related bacteria such as S. sobrinus spaA15.
The objective of this study was to detect S. mutans via the PCR that is specific to the spaP gene in samples of bacterial plaque from 56 mother/child pairs, both with and without dental caries, and identify if there was transmission of cariogenic bacteria between the pairs.
METHODS
Study population
The study population comprised 56 mother/child pairs from 11 Municipal Child Education Centers (CEMEIs) in the city of Londrina, Paraná, randomly selected out of a total of 46 CEMEIs. This project was submitted for the assessment of the Ethics in Research Committee at the Northern Paraná University (UNOPAR) and for evaluation by the Department of Education in Londrina, Paraná. The pre-school children's parents/guardians were informed about the nature of the study and also the need to obtain authorization, in accordance with the Code of Professional Ethics and the guidance contained in the National Health Council's Resolution 196 of October 10, 1996, for research studies involving human beings. After an explanation of the risks and benefits of the procedures, all those involved signed a free and informed consent form authorizing the performance of an oral examination and collection of saliva.
Evaluation of oral conditions
The evaluation of the oral health conditions was based on experience of dental caries, observing the criteria defined by the World Health Organization18, via the indexes DMFT for the mothers and dmft for the children. The pattern of oral hygiene was evaluated via the Simplified Oral Hygiene Index19 for the mothers and O´Leary's plaque control index20 for the children. The examinations were conducted under natural light, in ambient conditions, with the aid of a clinical mirror and probe for the removal of debris.
The participants were recruited based on the following criteria: 1) resident in an area with an excellent level of fluoride in the water supply, 2) not having a systemic disease and 3) not having used medication for a period of 15 days prior to the study and they were grouped, in accordance with their caries experience, as follows: those with dmft=0 and DMFT=0 were classified as caries-free and those with dmft and DMFT greater than or equal to 1 as having experience of the disease. The severity of the caries for the mother/child pairs was categorized in three groups: low severity (DMFT/dmft between 1 and 2.9), moderate severity (DMFT/dmft between 3 and 3.9) and high severity of caries (DMFT/dmft >4).
Samples
Samples of dental plaque, from 56 mother/ child pairs, were collected by means of sterilized probes, transferred to microcentrifuge tubes containing BHI medium (Brain Heart Infusion) for the subsequent extraction of DNA and the detection of Streptococcus mutans via PCR.
Extraction of bacterial DNA from the dental plaque
The collected samples were homogenized in a BHI medium and incubated in anaerobic jars for 48 hours at 37ºC. The bacteria culture was centrifuged at 3,000 rpm for 10 minutes. The supernatant liquid was discarded and the cell pellet was washed twice in 500 ul of TE buffer (Tris-HCl 10 mM, EDTA 1 mM, pH 8). To this was added 20μL of lysozyme (2mg/ml) and 10μL of proteinase K (4 μg/ ml) and incubated for 60 minutes at 37ºC. Subsequently, 10uL of SDS 10% were added together with 7.3μL of NaCl (4M) and incubated for 10 minutes at 60ºC. To the DNA precipitation was added ice-cold absolute alcohol. After the drying of the DNA, this was placed in 50μL of TE.
Amplification of specific S. mutans genes using PCR
The amplification of the 192 bp fragment of the spaP gene was carried out using PCR as described by Ono et al.15 using a thermal cycler (TC020A - Labnet International). In 25μL of reaction mixture there was: 200μM ddNTPs, 2.5 mM MgCl2, 2U Taq DNA polymerase, 25 pmol of each primer and 50 ng of DNA sample. For the spaP gene, the primers obtained from the Gene Bank (access no. x17390) were used, with the following sequence: Upstream 5'AAC GAC CGC TCT TCA GCA GAT ACC-3' / Downstream 5'AGA AAG AAC ATC TCT AAT TTC TTG-3'. The reaction mixture was denatured at 95ºC for 3 minutes; followed by a series of 30 amplification cycles: denaturation at 95ºC for 1 minute; pairing at 57ºC for 30 seconds and extension at 72ºC for 1 minute. The final cycle consisted of 94ºC for 1 minute; 55ºC for 1 minute and 72ºC for 5 minutes. For the positive control of the reaction, the amplification of 16s rDNA was used via the method described by Sato et al.21 and distilled water was used as the PCR negative control.
Electrophoresis in agarose gel
The amplicons were separated by electrophoresis in agarose gel (2%). The gel was dyed with ethidium bromide, viewed under ultraviolet light and photographed with a Nikon 5S digital camera. The size of the PCR product was estimated based on the electrophoretic migration relative to the molecular weight marker on the 100-bp Ladder (Invitrogen). Bands with a size of 192 bp (spaP) indicated the presence of S. mutans.
Statistical analysis
The SPSS (v. 15, SPSS Inc., Chicago) statistical package was used for the data analysis. Frequencies, averages and standard deviation were calculated for the sample description. The Chi-squared and Fisher's exact tests were carried out to test for the association between caries prevalence and the presence of cariogenic strains of S. mutans amongst the mother/child pairs. The correlation between caries experience in pre-school children and the presence of S. mutans spaP+ was evaluated via the Pearson correlation coefficient. The level of significance adopted for all analyses was p<0.05.
RESULTS
Root surface morphology
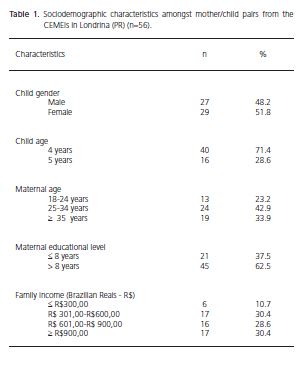
In the present study, there was a predominance of female children (51.8%) aged 4 (71.4%). A total of 42.9% of mothers were aged between 25 and 34. Around 60% of mothers had more than 8 years of schooling and family income between R$ 301 and R$ 900 (Table 1).
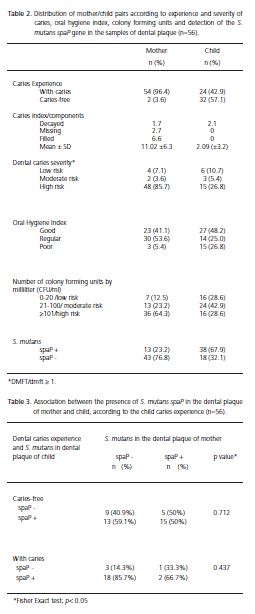
Amongst the mothers examined, experience of dental caries was 96.49% and average DMFT was 11.02 (SD=6.3) and the most prevalent component in this index was the filled tooth. In children it was observed that 57.1% were caries-free, having an average dmft of 2.09 (SD=3.2) with the carious component making up the entire index (Table 2).
In the population studied, 85.7% of mothers and 26.8% of children exhibited a high severity of caries and approximately 40% of the mother/child pairs had a good oral hygiene index. When the colony forming units were quantified, it was found that 28.6% of children had a low risk of caries (0-20 CFU/ml) and 64.3% of mothers showed a high risk of developing this disease (≥ 101 CFU/ml) (Table 2).
The presence of the S. mutans spaP gene was detected in the oral cavity in the mother/child pairs. As can be seen in Table 2, 67.9% of children were carriers of potentially cariogenic strains of S. mutans (spaP+) and only 23.2% of mothers exhibited this strain. The analysis via PCR with the primers 16S rDNA, confirmed the presence of bacteria in all the samples of dental plaque evaluated in this study.
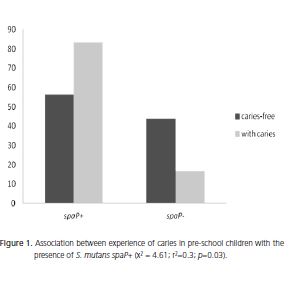
On analyzing the relationship between the experience of caries in children and sociodemographic variables, no statistical association was identified. However, a statistically significant association was identified between the presence of S. mutans carrying the spaP gene and experience of caries, but only in the children (Figure 1). The higher percentage of children with caries (83.3%) had S. mutans harboring the spaP+ gene (p=0.03) in their dental plaque.
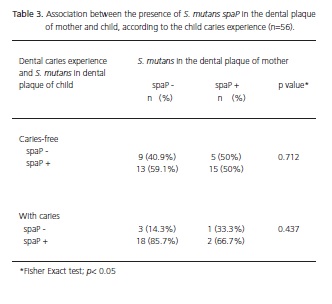
It can be seen from Table 3 that there was no association between the presence of S. mutans spaP+ in the dental plaque of mother and child. Only 10% of mothers harbored S. mutans spaP+ in their dental plaque while their children also had caries. For 90% of mothers who were negative (S. mutans spaP-) their children were carriers of S. mutans spaP+ and had dental caries.
DISCUSSION
The main etiological agent of dental caries, Streptococcus mutans, has developed multiple mechanisms for colonizing the tooth surface and, under certain conditions, it has become, numerically, the most significant specie in the cariogenic biofilm22. The multi-functional adhesin spaP, also known as P1 and PAc1, is considered to be the main factor in the initial fixation of S. mutans to the tooth enamel. The presence or absence of these bacteria could be a strong predictor of high or low susceptibility to dental caries23.
The results of this study showed that the prevalence of S. mutans spaP+ was greater in the children than in their mothers. Galaviz & Garcia24 also noted the presence of these bacteria in 74% of the children evaluated between the ages of 3 and 5.
The oral hygiene index for the children was deemed to be good. Although the majority of them had S. mutans spaP+ in their dental plaque, the average dmft evaluated was low (2.09±3.21). In addition to these findings, an association was found between experience of caries in children and the presence of the bacteria carrying the spaP+ gene. These data are in agreement with the study by Aguilera Galaviz et al.25 and Galaviz & Garcia24 who showed the presence of S. mutans with cariogenic potential to be more predominant than the accumulation of plaque. These researchers state that it is necessary to evaluate the dental plaque in qualitative terms, i.e. to detect the bacteria content and not just the number of bacteria. The presence of bacterial strains in the dental plaque carrying the spaP gene favors the development of dental caries, so it is therefore possible that S. mutans carries out adhesion by means of the interaction of the Ag I/II with the proteins of the acquired pellicle attached to the tooth24.
Recently, Duran-Contreras et al.26 investigated the relationship between the S. mutans spaP gene, present in the dental plaque, and dental caries. The authors found a strong association between the prevalence of caries in pre-school children and the high frequency of the S. mutans spaP gene. They found that, using the initiators for the spaP gene of the nucleic acids extracted from the S. mutans in the dental plaque, 91.3% of cases were S. mutans spaP+. They emphasized that all the children with caries tested positive for the presence of the spaP gene and only 8.75% were negative, and this group included children caries-free. This outcome was also found in the present study where 83.3% of pre-school children were positive for the spaP gene and also had the disease.
The mothers had a high DMFT due to previous experience with caries, as the most prevalent element of this index was the filled tooth. A fact that is worthy of note is that the average age of the mothers was 31 and when they were children, they received dental treatment with a more cure-based approach. Despite the fact that today's dental practices focus on prevention, it was observed that in children the carious component was entirely responsible for the dmft index.
When offering an explanation for the relationship between the presence of S. mutans carrying the spaP gene, experience and severity of caries amongst mother/child pairs, certain limitations of this study should be taken into account. No cause and effect relationship can be deduced from a cross-sectional delineation study such as this one. Longitudinal delineations could increase our understanding of the determinants of dental caries. In addition, the results of this study should be analyzed with caution due to the relatively small sample size.
This study did not demonstrate a similarity between the content of the dental plaque in the mother/child pair. A high percentage of children whose mothers did not have S. mutans with spaP in their dental plaque, were carriers of S. mutans spaP+ and had carious disease. It may be suggested that there was no vertical transmission between the mother/child pair. The S. mutans present in the oral cavity of the mothers has been reported as the main source for the maternal transmission of these microrganisms27-30. However Kozai et al.5, Redmo Emanuelsson & Thornqvist6 demonstrated an alternative transmission path through other members of the same family, such as fathers. It should be pointed out that horizontal transmissibility has been reported in some studies, demonstrating the possibility of the pathogenic agent also being acquired outside the family environment7-8 such as pre-school settings, where children share objects such as toys, pacifiers and teethers. Public centers cater to a large part of the population, particularly those at lower socioeconomic levels. These institutions represent an important target for the development of caries control programs. It is therefore relevant to investigate the main transmission paths of S. mutans in these populations.
In addition, the detection of a sequence of the S. mutans spaP gene in samples of dental plaque through polymerase chain reaction is a method which provides a quick and precise evaluation directly from the plaque, without the need to use selective means for the growth of bacteria, thereby establishing a more direct and qualitative system for the control of the risk of dental caries.
CONCLUSION
An association was found between experience of caries in children and the presence of bacteria carrying the spaP+ gene. There was no association related to the presence of the spaP+ gene between the mother/child pair, which suggests the absence of vertical transmission.
Acknowledgements
The agency for research grants: Funadesp.
Collaborators
FJS PIERALISI, SM MACIEL, FB ANDRADE, JE GARCIA, RC POLI-FREDERICO participated in all phases of the preparation of the manuscript.
REFERENCES
1. Global goals for oral health by the year 2000. Int Dent J. 1982;32(1):74-7. [ Links ]
2. Bowden GH. Does assessment of microbial composition of plaque/saliva allow for diagnosis of disease activity of individuals? Community Dent Oral Epidemiol. 1997;25(1):76-81.
3. Li Y, Caufield PW. The fidelity of initial acquisition of Mutans Streptococci by infants from their mothers. J Dent Res. 1995;74():681-5. doi: 10.1177/00220345950740020901.
4. Emanuelsson IR, Li Y, Bratthall D. Genotyping shows different strains of mutans streptococci between father and child and within parental pairs in Swedish families. Oral Microbiol Immunol. 1998;13(5):271-7.
5. Kozai K, Nakayama R, Tedjosasongko U, Kuwahara S, Suzuki J, Okada M, et al. Intrafamilial distribution of Mutans Streptococci in Japanese families and possibility of father-tochild transmission. Microbiol Immunol. 1999;43(2):99-106.
6. Redmo Emanuelsson I, Thornqvist E. Genotypes of Mutans Streptococci tend to persist in their host for several years. Caries Res. 2000;34(2):133-9. doi: 10.1159/000016580.
7. Mattos-Graner RO, Li Y, Caufield PW, Duncan M, Smith DJ. Genotypic diversity of Mutans Streptococci in Brazilian nursery children suggests horizontal transmission. J Clin Microbiol. 2001;39(6):2313-6. doi: 10.1128/JCM.39.6.2313-2316.2001.
8. Tedjosasongko U, Kozai K. Initial acquisition and transmission of mutans streptococci in children at day nursery. J Dent Child. 2002;69(3):284-8.
9. Klein MI, Florio FM, Pereira AC, Höfling JF, Goncalves RB. Longitudinal study of transmission, diversity, and stability of Streptococcus mutans and Streptococcus sobrinus genotypes in Brazilian nursery children. J Clin Microbiol. 2004;42(10):4620-6. doi:
10.1128/JCM.42.10.4620-4626.2004. 10. 10. Beighton D, Russell RR, Whiley RA. A simple biochemical scheme for the differentiation of Streptococcus mutans and Streptococcus sobrinus. Caries Res. 1991;25(3):174-8. doi: 10.1159/000261363.
11. de Soet JJP, van Dalen J, Pavicic MJAMP, de Graaff J. Enumeration of mutans streptococci in clinical samples by using monoclonal antibodies. J Clin Microbiol. 1990;28(11):2467-72. doi:
12. Cangelosi GA, Iversen JM, Zuo Y, Oswald TK, Lamont RJ. Oligonucleotide probes for mutans streptococci. Mol Cell Probes. 1994;8(1):73-80. doi: 10.1006/mcpr.1994.1011.
13. Okada M, Soda Y, Hayashi F, Doi T, Suzuki J, Miura K , Kozai K. PCR detection of Streptococcus mutans and S. sobrinus in dental plaque samples from Japanese pre-school children J Med Microbiol. 2002;51(5):443-7.
14. Igarashi T, Yamamoto A, Goto N. PCR for detection and identification of Streptococcus sobrinus. J Med Microbiol. 2000;49(12):1069-74.
15. Ono T, Hirata K, Nemoto K, Fernandes EJ, Ota F, Fukui K. Detections os Streptococcus mutans by PCR amplification of spaP gene. J Med Microbiol. 1994;41(4):231-5. doi: 10.1099/00222615-41-4-231.
16. Lee SF, Progulske-Fox A, Bleiweis AS. Molecular cloning and expression of a Streptococcus mutans major surface protein antigen, P1 (I/II), in Escherichia coli. Infect Immun. 1988;56(8):2114-9.
17. Okahashi N, Sasakawa C, Yoshikawa M, Hamada S, Koga T. Cloning of a surface protein antigen gene from serotype c Streptococcus mutans. Mol Microbiol. 1989;3(2):221-8. doi: 10.1111/j.1365-2958.1989.tb01811.x.
18. World Health Organization. Oral health surveys: basic methods. 3rd ed. Geneva: World Health Organization; 1997.
19. Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7-13.
20. O'Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43(1):38. doi:10.1902/jop.1972.43.1.38.
21. Sato T, Hu JP, Ohki K, Yamaura M, Washio J, Matsuyama J, et al. Identification of mutans streptococci by restriction fragment length polymorphism analysis of polymerase chain reactionamplified 16S ribosomal RNA gens. Oral Microbiol Immunol. 2003;18(5):323-5. doi: 10.1034/j.1399-302X.2003.00095.x.
22. Burne RA. Oral streptococci.products of their environment. J Dent Res. 1998;77(3):445-52. doi: 10.1177/00220345980770030301.
23. Bowen WH, Schilling K, Giertsen E, Pearson S, Lee SF, Bleiweis A, et al. Role of a cell surface-associated protein in adherence and dental caries. Infect Immun. 1991;59(12):4604-9.
24. Galaviz LAA, Garcia ICE. Detección de uma secuencia Del gene spaP de Streptococcus mutans en muestras de placa dental mediante reacción en cadena de la polimerasa (PCR). Revv Adm. 2003;60(5):180-4.
25. Galaviz LAA, Medina Mdel CA, García ICE. Detection of potentically cariogenic strains of Streptococcus mutans using the polymerase chain reaction. J Clin Pediatr Dent. 2002;27(1):47- 51.
26. Duran-Contreras GL, Torre-Martinez HH, de la Rosa El, Hernández RM, de la Ganza Ramos M. spaP gene of Streptococcus mutans in dental plaque and its relationship with early childhood caries. Eur J Paediatr Dent. 2011;12(4):220-4.
27. Caufield PW, Cutter GR, Dasanayake AP. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res. 1993;72(1):37-45. doi: 10.1177/00220345930720010501.
28. Lindquist B, Emilson CG. Colonization of Streptococcus mutans and Streptococcus sobrinus genotypes and caries development in children to mothers harboring both species. Caries Res. 2004;38:95-103. doi : 10.1159/000075932.
29. Berkowitz RJ. Mutans streptococci: acquisition and transmission. Pediatr Dent. 2006;28(2):106-9.
30. Wakaguri S, Aida J, Osaka K, Morita M, Ando Y. Association between caregiver behaviours to prevent vertical transmission and dental caries in their 3-year-old children. Caries Res. 2011;45(3):281-6. doi: 10.1159/000327211.
 Endereço para correspondência:
Endereço para correspondência:
RC POLI-FREDERICO
e-mail: reginafrederico@yahoo.com.br
Received on: 6/12/2011
Final version resubmitted on: 6/7/2012
Approved on: 5/8/2012













