Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.10 no.4 Joinville Out./Dez. 2013
ORIGINAL RESEARCH ARTICLE
In vitro study of solubilization ability of bovine pulp tissue using different irrigating solutions
Luiz Henrique Teixeira Fernandes I; Kenner Bruno Miguita I; Carlos Eduardo da Silveira Bueno I; Alexandre Sigrist de Martin I
I School of Dentistry, São Leopoldo Mandic – Campinas – SP –Brazil
ABSTRACT
Introduction and Objective: The aim of this study was to evaluate in vitro the capacity of solubilization of bovine pulp tissue, promoted by the following auxiliary chemical solutions: 2.5% sodium hypochlorite at pH 9, 5.25 % sodium hypochlorite at pH 9, 2% chlorhexidine gel at pH 6, 17% EDTA at pH 7, and Smear-Clear®. Material and methods: A total of ten specimens of bovine pulp tissue, weighing 1.65 g each, were placed into flasks connected to a device developed for the study in order to reproduce irrigation dynamics. The flasks with the specimens received a volume of 80 ml of irrigating solution and the experimental times were 15, 30, 45 and 60 minutes. Results: The results were analyzed statistically by Kruskal-Wallis test to compare the different experiment times (15, 30, 45 and 60 minutes) of each irrigating solution. To compare the variation among the times of one solution, Kolmogorov-Smirlov test (Lilliefors) was used. Conclusion: Considering the results obtained and the limitations of the study, it can be concluded that 5.25% sodium hypochlorite exhibited the greatest solubilization ability, followed by 2.5% sodium hypochlorite, Smear-Clear®, 17% EDTA and 2% chlorhexidine gel.
Keywords: dental pulp; therapeutic irrigation; dissolution; sodium hypochlorite; chlorhexidine; EDTA.
Introduction
At the ending of the 19th century, the clinicians and researchers dedicated to root canal treatment clearly agreed on the necessity of cleaning, disinfection and shaping of the root canal system aiming to its further obturation. To reach this goal, instruments (hand and rotary files and burs) are employed to obtain the preparation of the root canal system together with auxiliary chemical solutions so that also the cleaning of variations within the root canal internal morphology is achieved 3.
Thus, biomechanical preparation comprises the association of the mechanical with the chemical stage. The mechanical action prepares, cleans, and shapes the root canal, while the chemical action acts on the components within the root canals in order to dissolve either the live or necrosed organic tissues, remove the debris, and eliminate the microorganisms. Currently, sodium hypochlorite at several concentrations has been the solution of choice for the treatment of root canal system; however, other solutions have been studied, always aiming to find that comprising all desirable requirements for an irrigant.
One of the main properties of the irrigant solutions is the ability of tissue dissolution. The aim of this study is to analyze the solubilization ability of different irrigants during the root canal treatment, especially Smear-Clear, an EDTA-T-based solution plus cetrimide, whose potentiality is little known.
Material and methods
To obtain the bovine pulp tissue, the bovine mandibles were collected immediately after slaughter. The teeth were extracted with the aid of a n. 17 forceps, placed into plastic bags containing saline solution (to maintain the physiologic features of the pulp), kept into Styrofoam box with a thin layer of ice (to maintain the teeth cooled) and sent to the laboratory. Then, the teeth were cut at their long axes and the root and crown portion were separated to expose the coronal pulp. With the aid of a size 10 K file, the pulp tissue was detached and removed from the dentinal walls. Each fragment was washed with saline solution. Thus, the study sample was obtained by placing the fragments onto a glass plate and with the aid of a millimetric ruler and scalpel, each fragment was standardized at 10 mm weighing 1.65 g each, totalizing 8,27g for all experimental groups.
The specimens were divided according to the following groups:
• Group I – 2.5% sodium hypochlorite at pH 9 (Fórmula e Ação Farmácia de Manipulação, São Paulo, SP), batch 141;
• Group II – 5.25% sodium hypochlorite at pH 9 (Fórmula e Ação Farmácia de Manipulação, São Paulo, SP), batch 122;
• Group III – 2% chlorhexidine gel at pH 6 (Fórmula e Ação Farmácia de Manipulação, São Paulo, SP), batch f1107I0011;
• Group IV – 17% EDTA at pH 7 (Fórmula e Ação Farmácia de Manipulação, São Paulo, SP), batch f0607I0011;
• Group V – Smear-Clear (Sybron-endo, Optimum, São Paulo, SP.), batch 2731300; EXPIRATION DATE: 2010-02;
• Group VI – distilled water, control group.
The solutions were kept in a dry, fresh place, under environment temperature to maintain their physical-chemical characteristics. To conduct the bovine pulp dissolution test, a 10-100ml collector was adapted by previously perforating both sides. At one side, a urethane tube was adapted; at the other side, another tube was connected to link the collector to the circulation pump (SB 160; aquarium pump) (figure 1). The pump was placed into a plastic flask inside water. The bovine pulp fragments previously weighed with the aid of a precision scale were placed into the collectors with the aid of a net so that they became total immersed in the irrigants.

A volume of 80 ml (the ideal amount to make the system work correctly) of the auxiliary chemical solution of each experimental group was used with a constant flow of 64 ml/min, and the following times: 15, 30, 45 and 60 minutes.
Elapsed the experimental times, the tissue remnants (when present) were washed in distilled water for 1 minute, dried with gauze, and weighed in the precision scale (Colemam). The results were expressed as the percentage of the initial weigh of the specimens.
Results
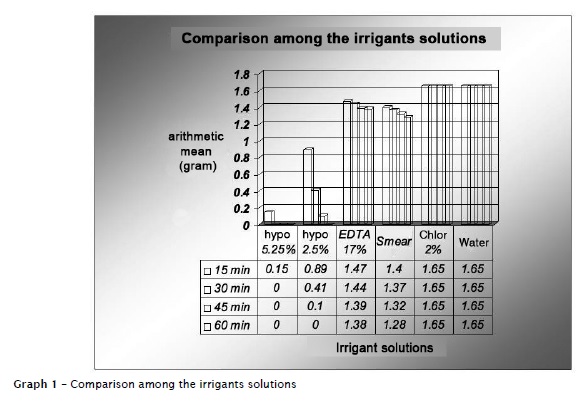
The samples exhibited an abnormal and homogenous behavior. Kruskal-Wallis test was applied to compare the different experimental times (15, 30, 45 and 60 minutes) of each irrigant solution, and, the different irrigant solutions at each time period. Kolmogorov-Smirlov test (Lilliefors) was applied to compare the variations among the time periods of each solution (graph 1).
Discussion
The collection of the bovine teeth to remove the pulp immediately after the slaughter aimed to achieve specimens with good physiologic features (texture, color, and hydration). The fast and careful pulp tissue preparation was a primordial step of the study, avoiding damages such as the excessive heating of the tissue.
The sodium hypochlorite solutions at 2.5% and 5.25% concentrations, 17% EDTA, and 2% chlorhexidine gel were obtained from the same manufacturer (manipulation pharmacy Fórmula & Ação), aiming to standardization. The solutions were kept at environmental temperature, protected from light, to maintain their physical-chemical characteristics. The washing of the specimens between the periods tested for all solutions was carried out to avoid an additional dissolution – even minimum – during the turning off of the system and the transportation to the sink, especially for 2.5% and 5.25%, hypochlorite solutions. Thus, the scale and the device were strategically placed by the side of the sink to assure a fast weighing of the specimens. For each group, the procedures were repeated eight times to allow the statistical analysis.
For each evaluation period, the auxiliary chemical substance was changed to avoid contamination during the measurements by inserting 80 ml of saline solution. Then, the system was started and let to work for the aforementioned purpose.
The use of chemical substances during the root canal instrumentation is of fundamental importance. The cut dentine and the pulp remnants should be encompassed by the chemical solution to prevent the smear layer formation and its deposition onto the apical portion of the canal, therefore obstructing it. Additionally, these substances should exhibit the following characteristics: make the use of the instruments easy; remove the organic remnants, contaminated or not; act against the possible existing microorganisms.
Considered the results obtained, the sodium hypochlorite was the solution with the greatest solubilization ability of the bovine pulp tissues, at a shorter time period compared with the other solutions tested. These results corroborates the findings observed by Walker 32, Grossman and Meiman 11, Hand et al. 12, Rosenfeld et al. 28, Thé et al. 31, Koskinen et al. 16, Abou-Rass and Oglesby 1, Gordon et al. 10, Moorer and Wesselink 21, Nakamura et al. 24, Hasselgren et al. 13, Morgan et al. 22, Andersen et al. 2 and Johnson and Remeikis 14. The tissue dissolution action promoted by sodium hydroxide, one of the byproducts of sodium hypochlorite is a powerful organic and fat solvent 21. Another byproduct – the hypochlorous acid – is a powerful antimicrobial agent because it releases chlorine which combines with the amino group from proteins, forming the chloramines. The hypochlorous acid (HOCl) undergoes decomposition by light, air and heat action releasing free chlorine and secondarily oxygen; also depending on other factors such as: concentration, temperature, hydrogen potential, volume, contact time, contact surface and pulp state 1,10,12,21,23,30.
Siqueira et al. 29 reported that the 2.0% chlorhexidine gel and 0.5% hypochlorite solutions at pH 7, ranging from 27ºC to 37ºC were not effective in dissolving organic tissues. It is important to emphasize the greater solubilization ability of Smear-Clear than that of 17% EDTA, despite the fact that they have similar formulations (same active principle). Studies previously conducted indicating the lower ability of this solution in dissolving pulp tissue 4-6,9,15,25,26,34, primarily because of the pH of EDTA, around 7 17. In this present study, 17% EDTA exhibited small ability of tissue dissolution.
According to the manufacturer, Smear-Clear® is an adjunct solution for the final endodontic irrigation to promote the refining in the ending of the chemical-mechanical preparation, mainly as an auxiliary solution for smear layer solution, as verified by the study of Lui et al. 19. Although its main function is to remove the smear layer, the results obtained by this present study suggested the presence of an important property: the ability of dissolving pulp tissue. As basic formulation, Smear-Clear (Sybron-endo) contains 17% EDTA plus a detergent agent (to improve the cleaning properties) and cetrimide (an antiseptic agent with bactericidal function). Chlorhexidine seems to exhibit some advantages in relation to sodium hypochlorite, such as substantivity 8,18,33 and its antimicrobial potential 8,7,18,27. One of its greatest disadvantages is the small ability to dissolve organic tissue, as observed by this present study corroborating the findings of D'Arcangelo et al. 7 and Marley et al. 20.
Conclusion
Within the limits of this study and based on the results obtained, it can be concluded that 5.25% sodium hypochlorite exhibited the greatest solubilization ability of bovine pulp tissue, followed by 2.5% sodium hypochlorite, Smear-Clear®, 17% EDTA, and 2% chlorhexidine gel.
References
1. Abou-Rass M, Oglesby SW. The effects of temperature, concentration, and tissue type on the solvent ability of sodium hypochlorite. J Endod. 1981;7(8):376-7. [ Links ]
2. Andersen M, Lund A, Andreasen JO, Andreasen FM. In vitro solubility of human pulp tissue in calcium hydroxide and sodium hypochlorite. J Endod. 1992;8(3):104-8.
3. Arruda MP, Sousa YT, Correa S, Cruz-Filho AM, Souza-Filho FJ, Sousa-Neto MD. Análise histológica da capacidade de limpeza promovida pela instrumentação rotatória com limas de níquel-titânio, em canais radiculares com achatamento mésio-distal, utilizando diferentes soluções químicas auxiliares do preparo biomecânico. J Bras Endod. 2003;4(13):141-7.
4. Baumgartner JC, Mader CL. A scanning electron microscopic evaluation of four root canal irrigation regimens. J Endod. 1987;13(4):147-57.
5. Beltz RE, Torabinejad M, Pouresmail M. Quantitative analysis of the solubilizing action of MTAD, sodium hypochlorite, and EDTA on bovine pulp and dentin. J Endod. 2003;29(5):334-7.
6 . Cury JA, Bragotto C, Valdrighi L. The demineralizing efficiency of EDTA solutions on dentin. I. Influence of pH. Oral Surg Oral Med Oral Pathol. 1981;52(4):446-8.
7. D'Arcangelo C, Varvara G, De Fazio P. An evaluation of the action of different root canal irrigants on facultative aerobic-anaerobic, obligate anaerobic, and microaerophilic bacteria. J Endod. 1999;25(5):351-3.
8. Denaly GM, Patterson SS, Miller CH, Newton CW. The effect of chlorhexidine gluconate irrigation on the root canal flora of freshly extracted necrotic teeth. Oral Surg Oral Med Oral Pathol. 1982;53(5):518-23.
9. Giardino L, Ambu E, Becce C, Rimondini L, Morra M. Effect of EDTA with and without surfactants or ultrasonics on removal of smear layer. J Endod. 2006;32(11):1091-3.
10. Gordon TM, Damato D, Christner P. Solvent effect of various dilutions of sodium hypochlorite on vital and necrotic tissue. J Endod. 1981;7(10):46666-9.
11. Grossman LI, Meiman BW. Solution of pulp tissue by chemical agents. J Am Dent Assoc. 1941;28(2):223-5.
12. Hand RE, Smith ML, Harrison JW. Analysis of the effect of dilution on the necrotic tissue dissolution property of sodium hypochlorite. J Endod. 1978;4(2):60-4.
13. Hasselgren G, Olsson B, Cvek M. Effects of calcium hydroxide and sodium hypochlorite on the dissolution of necrotic porcine muscle tissue. J Endod. 1988;14(3):125-7.
14. Johnson BR, Remeikis NA. Effective shelf-life of prepared sodium hypochlorite solution. J Endod. 1993;19(1):40-3.
15. Jussila O, Pohto M. The widening of narrow root canals by chemical means. Suom Hammaslaak Toim. 1954;50(2):122-32.
16. Koskinen KP, Meurman JH, Stenvall H. Appearance of chemically treated root canal walls in the scanning electron microscope. Scand J Dent Res. 1980;88(5):397-405.
17. Kuah HG, Lui JN, Tseng PS, Chen NN. The effect of EDTA with and without ultrasonics on removal of the smear layer. J Endod. 2009;35(3):393-6.
18. Kuruvilla JR, Kamath MP. Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined, as endodontic irrigants. J Endod. 1998;24(7):472-6.
19. Lui JN, Kuah HG, Chen NN. Effect of EDTA with and without surfactants or ultrasonics on removal of smear layer. J Endod. 2007;33(4):472-5.
20. Marley JT, Ferguson DB, Hartwell GR. Effects of chlorhexidine gluconate as an endodontic irrigant on the apical seal: short-term results. J Endod. 2001;27(12):775-8.
21. Moorer WR, Wesselink PR. Factors promoting the tissue dissolving capability of sodium hypochlorite. Int Endodontic J. 1982;15(4):187-96.
22. Morgan RW, Carnes Jr. DL, Montgomery S. The solvent effects of calcium hydroxide irrigating solution on bovine pulp tissue. J Endod. 1991;17(4):165-8.
23. Naenni N, Thoma K, Zehnder M. Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J Endod. 2004;30(11):785-7.
24. Nakamura H, Asai K, Fujita H, Nakazato H, Nishimura Y, Furuse Y et al. The solvent action of sodium hypochlorite on bovine tendon collagen, bovine pulp, and bovine gingiva. Oral Surg Oral Med Oral Pathol. 1985;60(3):322-6.
25. Nikiforuk G, Sreebny L. Demineralization of hard tissues by organic chelating agents at neutral pH. J Dent Res. 1953;32(6):859-67.
26. O'Connell MS, Morgan LA, Beeler WJ, Baumgartner JC. A comparative study of smear layer removal using different salts of EDTA. J Endod. 2000;26(12):739-43.
27. Ringel AM, Patterson SS, Newton CW, Miller CH, Mulhern JM. In vivo evaluation of chlorhexidine gluconate solution and sodium hypochlorite solution as root canal irrigants. J Endod. 1982;8(5):200-4.
28. Rosenfeld EF, James GA, Burch BS. Vital pulp tissue response to sodium hypochlorite. J Endod. 1978;4(5):140-6.
29. Siqueira EL, Santos M, Bombana AC. Dissolução de tecido pulpar bovino por duas substâncias químicas do preparo do canal radicular. RPG. 2005;12(3):316-22.
30. Sirtes G, Waltimo T, Schaetzle M, Zehnder M. The effects of temperature on sodium hypochlorite short-term stability, pulp dissolution capacity, and antimicrobial efficacy. J Endod. 2005;31(9):66669-71.
31. Thé SD, Maltha JC, Plasschaert AJ. Reactions of guinea pig subcutaneous connective tissue following exposure to sodium hypochlorite. Oral Surg Oral Med Oral Pathol. 1980;49(5):460-6.
32. Walker A. A definite and dependable therapy for pulpless teeth. J Am Dent Assoc. 1936;23(2):1418-25.
33. Wang CS, Roland R, Arnold RR, Trope M, Teixeira FB. Clinical efficiency of 2% chlorhexidine gel in reducing intracanal bacteria. J Endod. 2007;33(11):1283-9.
34. Yamada RS, Armas A, Goldman M, Lins PS. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: part 3. J Endod. 1983;9(4):137-42.
 Corresponding author:
Corresponding author:
Kenner Bruno Miguita
Rua Monteiro de Barros, n. 90 – Centro
CEP 13280-000 – Vinhedo – SP – Brasil
E-mail: kennerbm@uol.com.br
Received for publication: April 23, 2013
Accepted for publication: June 27, 2013













