Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RSBO (Online)
versão On-line ISSN 1984-5685
RSBO (Online) vol.11 no.4 Joinville Out./Dez. 2014
ORIGINAL RESEARCH ARTICLE
Influence of the powder/liquid ratio and storage time of conventional glass ionomer cements in diametral tensile strength
Tamiris Carolina da Silva I; Camila Rodrigues dos Santos I; Rodrigo Borges Fonseca I; Terezinha Jesus Esteves Barata I
I Department of Oral Prevention and Rehabilitation, School of Dentistry, Federal University of Goiás – Goiânia – GO – Brazil
ABSTRACT
Introduction: The Glass Ionomer Cements (GICs) are the most versatile dental material with extensive clinical indication. However, the mechanical strength of conventional GICs (C-GICs) is still unsatisfactory under areas of masticatory forces. Objective: To evaluate the influence of C-GIC proportioning system (powder-liquid and encapsulated) and storage period (1h, 24h and 7 days) on Diametral Tensile Strength (DTS). Material and methods: The two variables were tested in relation to C-GIC (Riva Self Cure, SDI, Australia). The following proportioning systems were tested: powder-liquid by weight (g:g) and powder-liquid by volume (flat scoop: drop) and encapsulated system (pre-dosed capsules). Five C-GIC specimens were prepared, according to ISO specification #9917 for each variable to be studied. The specimens were stored in plastic containers containing distilled water and kept at 37ºC and 100% humidity until the mechanical testing in a universal testing machine (Instron Corp., USA) at a speed 0.5 mm/min. The data were submitted to two-way ANOVA and Tukey test for multiple comparisons (α = 0.05). Results: The proportioning system (P < 0.0001) and storage time (P < 0.0001) were significant predictors of DTS, however with no interaction between these factors. Conclusion: The DTS of C-GICs was influenced by storage time (1 hour < 24 hours < 7 days) and by the proportioning system only for the initial period of its setting reaction.
Keywords: dental research; glass ionomer cements; material resistance.
Introduction
Over the last four decades, glass ionomer cements (GICs) experienced a period of constant technological advancements 9,10,14,15,17, thus becoming an excellent dental material preventive and even restorative option in some specific situations 10,11. Thus, the main GIC clinical indications are: caries sealing for oral adequacy, pit and fissure sealants, conservative direct restorative procedures in primary and permanent teeth, and protection of dentin-pulp complex 10,13,16. Moreover, GICs are still successfully indicated in orthodontic, endodontic, and prosthetic procedures 10,16.
The large GICs clinical indication is directly related to the positive properties as f luoride releasing and uptake, adhesivity, biocompatibility, coefficient of linear thermal expansion similar to that of the tooth structures 10,13. On the other hand, conventional GICs have critical points related to their longer setting time, sensitivity to humidity, esthetics, higher superficial roughness, unsatisfactory mechanical resistance under areas of masticatory efforts 8,10,13,16. Within this context, it is noteworthy to mention that the incorporation of porosities to GICs during the proportion, handling and/or insertion will lead to decrease the mechanical resistance 1,6,18. The rationale behind this fact is that internal CIG porosities may cause local stress and result in crack propagation and partial or total fracture of the restoration, because the porosities may be located inside the restoration and/or at tooth/restoration interface 1,6,18.
Accordingly, Chammas et al. 4 inferred that encapsulated GICs would have less porosity inclusion because they are both proportioned and mixed mechanically and inserted with the aid of a specific device for application. It is important to highlight that the storage time may interfere in GICs mechanical resistance 1,3, because the maturat ion process of these materials is gradual and occur within the f irst 24 hours after mixing 18. These continuous reactions cause the increasing of the mechanical properties 1,3. Thus, it should be highlighted the importance of studies evaluating the inf luence of GICs proport ioning system (encapsulated system [pre-dosed capsules] x powder-liquid system) and storage period on mechanical resistance. Thus, the present study aimed to evaluate the inf luence of C-GIC proportioning system (powder-liquid by weight [g:g]; powder-liquid by volume [f lat scoop:drop]; and encapsulated [pre-dosed capsules]), and the storage period on diametral tensile strength (DTS). The null hypothesis to be tested is that both the proport ioning system and storage time would not inf luence DTS if the operator is properly trained.
Material and methods
Two proportioning systems of conventional restorative glass ionomer cement (Riva Self Cure) were tested: powder-liquid and pre-dosed capsules (table 1).
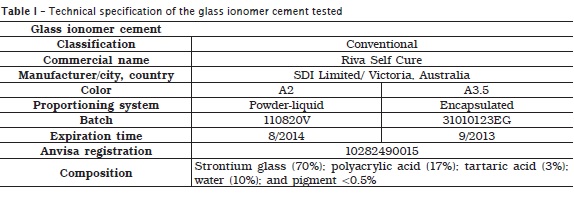
Study design
The study sample was divided into three groups according to figure 1.
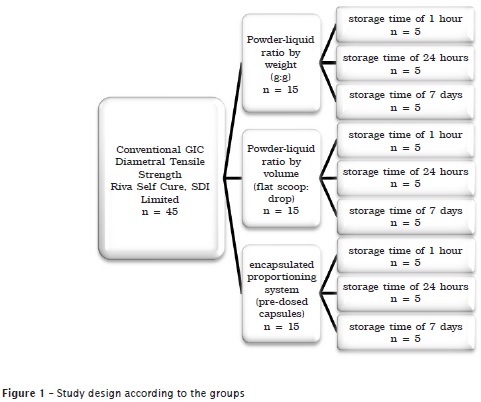
Five specimens were constructed with the aid of stainless steel matrix (6±0.1 mm in diameter and 3±0.1 mm in height), for each variable studied, and according to ADA specification no. #66 1, at a laboratory with controlled environmental temperature (23±1ºC) and relative humidity (50±5%).
Powder-liquid system groups
Two operators properly trained and calibrated constructed the specimens, so that one operator was responsible for CIG powder-liquid ratio and the other for GIC handling and insertion. For the group of powder-liquid ratio by weight (g:g), an analytical precision scale (Mater, AY220, Santa Rita do Sapucaí, MG, Brazil) was used to measure the weight. This allowed the accurate standardization of powder-liquid ratios, aiming to avoid volumetric alterations.
For the group of powder-liquid ratio by volume, the flat scoop and dropper provided by the manufacturer were used, according to the manufacturer's instructions. For both groups, GIC mixing was performed according to the manufacturer's instructions. Next, GIC was inserted into the stainless steel matrix previously covered by solid Vaseline (Rioquímica Indústria Farmacêutica, São José do Rio Preto, SP, Brazil) with the aid of an insertion syringe (Centrix, Shelton, CT, USA). Immediately after GIC insertion, a polyester strip (TDV Dental, São Paulo, SP, Brazil) was placed over the material and a glass lamina (Glasstécnica, São Paulo, SP, Brazil) was pushed against the specimen with the aid of 250 g load. After two minutes from the mixing onset, the matrix/specimen set was placed into an incubator at 37±1°C until completing 15 minutes from mixing.
Elapsed that time, the specimen were carefully removed from the matrix and placed into an individual plastic flask containing distilled water. The specimens were stored into the incubator (Mettler Toledo, Sanford, USA) until the test periods of 1 hour, 24 hours, and 7 days.
Encapapsulated group
In this group, the specimens were also constructed by two operators previously trained and calibrated, so that one operator was responsible for the mechanical mixing and the other for GIC insertion. A digital amalgamator Ultramat 2, SDI Limited, Victoria, Australia) was used to mix mechanically the GIC capsules during 10 seconds. Next, GIC insertion was executed similarly to the aforementioned groups, except that GIC was inserted with the aid of the manufacturer's applicator (Riva Applicator, SDI Limited, Victoria, Australia).
Mechahanical tests
DTS mechanical tests were carried out in an universal testing machine (Instron 4411; Instron Testing Instruments, Canton, MA, USA), with crosshead speed of 0.5 mm/min, at the storage periods of 1 hour, 24 hours, and 7 days, counting from the beginning of GIC mixing by an operator properly trained.
Statistical analysis
The obtained data were submitted to descriptive statistics, normality test, and two-way ANOVA, followed by Tukey test. All analyses were performed with the aid of SPSS 11.0 software (SPSS Inc., Chicago, IL, USA), with level of significance of 0.05 (P < 0.05).
Results
Kolmogorov-Smirnov normality test suggested a normal distribution of the results (P > 0.05). Two-way ANOVA results indicated no interaction between the factors evaluated (proportioning system x storage time) (P = 0.364), and showed that both the proportioning system (P < 0.0001) and storage time (P < 0.0001) were significant predictors of DTS.
The DTS mean and standard deviation in function of GIC proportioning system at the three storage times are displayed in table 2.
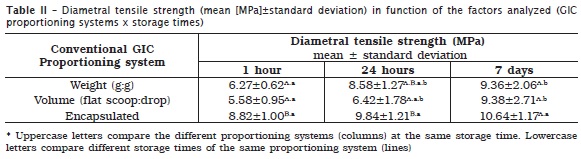
The analysis of the data observed that DTS increased in function of storage time, regardless of the proportioning system: 1 hour < 24 hours < 7 days.
The results evidenced a statistically significant difference (Tukey test) for the proportioning system by weight (g:g) (P = 0.015) between the storage period of 1 hour and 7 days (P = 0.014), while the period of 24 hours was statistically similar to the period of 1 hour (P = 0.065) and 7 days of storage (P = 0.068).
Moreover, statistically significant differences were observed for the proportioning system by volume (flat scoop:drop) only between the storage time of 1 hour and 7 days (P = 0.024), while the period of 24 hours was statistically similar to the other periods (1 hour and 7 days).
No statistically significant differences were observed at all tested periods (1 hour, 24 hours, and 7 days) for the proportioning system by pre-dosed capsules. Based on the obtained results, the null hypothesis was partially accepted because the proportioning system only influenced DTS at the initial setting time (1 hour and 24 hours).
Discussion
The results of this study revealed that GIC proportioning system influenced DTS at the initial moments of the setting time because the encapsulated system showed higher tensile strength than did powder-liquid system. Also, at the period of 24 hours, there were statistically significant differences between the proportioning system by powder-liquid by weight and by pre-dosed capsules. After 7 days, the three proportioning systems did not show statistically significant differences regarding DTS.
It is noteworthy to highlight that regardless of the proportioning system tested, DTS value increased according to the storage period (1 hour < 24 hours < 7 days), which agrees with the literature 1,3,6. It could be inferred that DTS increasing is directly related to the acid-base setting reaction and maturation process of C-GIC. Additionally, this present study found that regardless of the storage period tested (1 hour, 24 hours and 7 days) and the presence or absence of statistically significant differences, DTS followed the same pattern: encapsulated system > powder-liquid system by weight > powder-liquid system by volume. This pattern can be attributed to the fact that GIC mechanical properties are strictly related to its micro-structure 18. Accordingly, the integrity between the glass particles and the matrix, the particle size and the number of porosities incorporated into the material play an important role in the mechanical properties 18.
Thus, the present study is representative because we use the same C-GIC and the composition did not influence DTS result. Molina et al. 12 evaluated three different GIC brands in two different presentations: pre-dosed capsules (Fuji 9 Gold Label/GC Europe and Ketac Molar Easy Mix/3M ESPE) and powder-liquid ratio (ChemFil Rock/Dentsply DeTrey GmbH; EQUIA system Fuji GP Extra + G-Coat/GC Asia). These authors concluded that diametral, compression and flexural tensile strengths of pre-dosed capsules were significantly higher than those of powder-liquid GIC 12.
The study of Dowling and Fleming 5 affirmed that the compressive strength, modulus of elasticity, and resistance to wear of pre-dosed C-GIC capsules were significantly higher than those of powder-liquid GIC, when the powder content was smaller than that recommended by the manufacturer 5. Similarly, Fonseca et al. 7 also advocated that the reduction of the powder-liquid ratio affects the properties of C-GICs and resin-modified GICs 7.
In this study the operators were properly trained and calibrated regarding the proportioning and mixing of the powder-liquid presentation and the insertion of the two proportioning systems used. Therefore, we hypothesized that DTS should not be influenced by the proportioning system. Notwithstanding, we observed that the proportioning system did influence only the initial performance with higher DTS values for encapsulated GICs and statistically similarity between the both modes of powder-liquid proportioning (weight and volume). The results obtained in this study indicated the importance of previous training of the dental team and both the weight and volume proportioning of powder-liquid system are equivalent since the operators are properly trained. One should recognize that the variability of the operators during GIC proportioning of GIC powder-liquid may directly affect the performance 5,6,7,12,18.
Dowling and Fleming 5 still alerted that the improper proportioning of powder-liquid system, that is, smaller powder amount than that recommended by the manufacturer, would not be an uncommon practice in daily clinical routine 5. Accordingly, Molina et al. 12 affirmed that GIC (powder-liquid system) performance is dependent on the extrinsic variabilities, such as: proportioning, amount of porosities incorporated during mixing, and variables related to the insertion of these materials. The authors still stated that encapsulated GICs would cancel the effect of the extrinsic variabilities related to this material 12. The results of this study can corroborate the affirmations Molina et al. 12 by stating that the clinical use of encapsulated GICs would minimize the extrinsic variability.
Conclusion
• The increase of C-GIC DTS was dependent on the storage time (1 hour < 24 hours < 7 days);
• Regardless of the storage time tested (1 hour, 24 hours, and 7 days), DTS followed a similar strength pattern: encapsulated system > powder-liquid proportioning system by weight > powder-liquid proportioning system by volume. Therefore the proportioning system influenced DTS only at the initial period of the setting reaction.
References
1. American Dental Association, Specification n. 66 for dental glass ionomer cements. Council on Dental Materials, Instruments and Equipment. JADA. 1989;119:205. [ Links ]
2. Barata TJE, Bresciani E, Adachi A, Fagundes TC, Carvalho CAR, Navarro MFL. Influence of ultrasonic setting on compressive and diametral tensile strengths of glass ionomer cements. Mat Res. 2008 Mar;11(1):57-61.
3. Bresciani E, Barata TJE, Fagundes TC, Adachi A, Terrin MM, Navarro MFL. Compressive and diametral tensile strength of glass ionomer cements. JMID. 2008;1(2):102-11.
4. Chammas MB, Valarini N, Maciel SM, Poli-Frederico RC, Oltramari-Navarro PVP, Conti ACCF. Resistência à compressão de cimentos de ionômero de vidro restauradores encapsulados. UNOPAR Cient Ciênc Biol Saúde. 2009;11(4):35-8.
5. Dowling AH, Fleming GJ. Are encapsulated anterior glass-ionomer restoratives better than their hand-mixed equivalents? J Dent. 2009 Feb;37(2):133-40.
6. Esteves Barata TJ, Bresciani E, Cestari Fagundes T, Gigo Cefaly DF, Pereira Lauris JR, Lima Navarro MF. Fracture resistance of class II glass-ionomer restorations. Am J Dent. 2008;21:163-7.
7. Fonseca RB, Branco CA, Quagliatto PS, Gonçalves LS, Soares CJ, Carlo HL et al. Influence of powder/liquid ratio on the radiodensity and diametral tensile strength of glass ionomer cements. J Appl Oral Sci. 2010 Dec;18(6):577-84.
8. Freitas MFA, Imai LJ, Freitas CA, Bianchi EC, Almeida CT, Martins Filho IE. Abrasive wear of two glass ionomer cements after simulated toothbrushing. RSBO. 2011 Jul-Sep;8(3):287-93.
9. Guggenberger R, May R, Stefan KP. New trends in glass-ionomer chemistry. Biomaterials. 1998 Mar;19(6):479-83.
10. Khoroushi M, Keshani F. A review of glass-ionomers: from conventional glass-ionomer to bioactive glass-ionomer. Dent Res J (Isfahan). 2013 Jul;10(4):411-20.
11. Mickenautsch S. How well are GIC product labels related to current systematic review evidence? Dent Update. 2011 Nov;38(9):634-8.
12. Molina GF, Cabral RJ, Mazzola I, Lascano LB, Frencken JE. Mechanical performance of encapsulated restorative glass-ionomer cements for use with atraumatic restorative treatment (ART). J Appl Oral Sci. 2013;21(3):243-9.
13. Mount GJ, Tyas MJ, Ferracane JL, Nicholson JW, Berg JH, Simonsen RJ et al. A revised classification for direct tooth-colored restorative materials. Quintessence Int. 2009 Sep;40(8):691-7.
14. Perdigão J, Dutra-Corrêa M, Saraceni S, Ciaramicoli M, Kiyan V. Randomized clinical trial of two resin-modified glass ionomer materials: 1-year results. Oper Dent. 2012 Nov-Dec;37(6):591-601.
15. Sidhu SK. Clinical evaluations of resin-modified glass-ionomer restorations. Dent Mater. 2010 Jan;26(1):7-12.
16. Vaderhobli RM. Advances in dental materials. Dent Clin North Am. 2011 Jul;55(3):619-25.
17. Wilson AD, Kent BE. A new translucent cement for dentistry. The glass ionomer cement. Br Dent J. 1972 Feb;132(4):133-5.
18. Xie D, Brantley WA, Culbertson BM, Wang G. Mechanical properties and microstructures of glass-ionomer cements. Dent Mater. 2000 Mar;16(2):129-38.
 Corresponding author:
Corresponding author:
Terezinha Jesus Esteves Barata
Av. Universitária, Esquina com 1.ª Avenida, s.n. – Setor Universitário
CEP 74605-220 – Goiânia – GO – Brasil
E-mail: terezinhabarata@yahoo.com.br
Received for publication: March 31, 2014
Accepted for publication: September 1, 2014













