Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Revista Brasileira de Odontologia
versão On-line ISSN 1984-3747versão impressa ISSN 0034-7272
Rev. Bras. Odontol. vol.73 no.4 Rio de Janeiro Out./Dez. 2016
Review Literature Article/ Basic Sciences
Dental stem cells and their application in Dentistry: a literature review
Paula Nascimento AlmeidaI,II; Karin Soares CunhaI, II
I Programa de Pós-Graduação em Patologia, Faculdade de Medicina, Universidade Federal Fluminense, Niterói, Rio de Janeiro, Brasil
II Centro Nacional de Neurofibromatose, Rio de Janeiro, Rio de Janeiro, Brasil
ABSTRACT
Objective: the aim of this study was to conduct a literature review of the types of stem cells of dental origin and their applications in Dentistry. Material and Methods: for this, we selected scientific articles published between 2000 and 2016 through the databases PUBMED and LILACS. Results: there are five main sources of stem cells of dental origin: stem cells from dental pulp of permanent teeth and deciduous teeth, apical papilla, periodontal ligament and dental follicle. These cells have been studied for the treatment of periodontitis, bone repair, regeneration of the pulp after necrosis as well as the development of new teeth. Conclusion: stem cells from dental origin are an interesting alternative for research and application in regenerative therapies in Dentistry.
Descriptors: Stem cells; Tissue engineering; Dentistry.
Introduction
Stem cells (SCs) are undifferentiated cells with self-renewal ability and capacity to differentiate into specialized cell types.1,2 Regarding the origin, they can be classified as embryonic stem cells (ESCs) and adult stem cells (ASCs).3 Embrionic stem cells (ESCs) are derived from the inner cell mass of the blastocyst and form all cell types, derived from the three germ layers, and are therefore pluripotent.3,4 The zygote and cells derived from the first two cellular divisions constitute the most primitive cells (totipotent cells) that are capable of forming the embryo and the embryonic annexes (e.g. placenta, amniotic membranes etc).
ASCs are present in a number of posnatal tissues and are responsible for normal tissue renewal as well as for regeneration and healing after injuries. Due to the ability to self-renew and to differentiate into cells that are found throughout the body, there is a great interest in using stem cells for the regeneration of injured tissues as well as to develop tissue-engineered implants and bio-hybrid organs, in order to restore tissue function. The use of ASCs in regenerative medicine and tissue engineering research has important advantages in comparison with ESCs, since there are no ethical complications and the process of differentiation of these cells is better controlled.5
Mesenchymal stem cells (MSCs) are ASCs, and were first described in 1966 by Friedenstein et al.6 Since then, clinical and biological interest in MSCs have increased and the Mesenchymal Stem Cells Committee of the International Society for Cellular Therapy proposed a minimum criteria for the identification of these cells: adherence to plastic culture surfaces, potential of osteogenic, adipogenic and chondrogenic differentiation in vitro as well as expression of surface antigens CD73, CD90 and CD105 and lack of expression of hematopoietic and endothelial markers CD14 or CD11b, CD34, CD45, CD79alpha or CD19 and human leukocyte antigen-DR (HLA-DR).7
MSCs can be isolated from different locations, such as bone marrow, umbilical cord, placenta, adipose and dental tissues. 8,9 Because dental stem cells (DSCs) are easy to obtain and present a great potential of differentiation, there has been a growing interest in their use in regenerative medicine for treatment of various human diseases.10
In human postnatal dental tissues, five main sources of DSCs have been identified: dental pulp stem cells (DPSCs),11 stem cells from human exfoliated deciduous teeth (SHEDs),12 periodontal ligament stem cells (PDLSCs),13 dental follicle stem cells (DFSCs)14 and stem cells from apical papilla (SCAPs).15 Regardless the tooth tissue of origin, DSCs can be isolated either disaggregating the tissue enzymatically and/or mechanically, or also by explant. After enzymatic and/or mechanical dissociation, cells are placed in culture medium for growing on plastic flasks or dishes. In explant method, the dental tissue is placed on a plastic surface and the cells migrate out from the tissue fragment adhering to culture flasks or dishes (Figure 1).
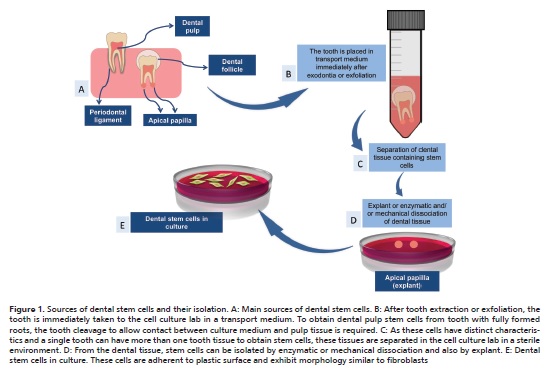
Specific properties, such as proliferative and differentiation potential, are slightly different among DSCs. Thus, the aim of this study was to conduct a literature review of DSCs and their applications in Dentistry.
Material e Methods
Scientific papers published between 2000 and 2016 were selected through PUBMED (Public Medicine) and LILACS (Latin American and Caribbean Health Sciences) databases. The key words used were: "dental stem cells"; "stem cells" AND "application" AND "dentistry"; "dental stem cells" AND "tissue engineering". Full texts of the articles written in Portuguese and English were included. Papers that did not directly address the subject were excluded.
Results
•Dental Pulp Stem Cells
DPSCs were first isolated from human third molars by Gronthos et al.11 These cells express surface markers similar to those of MSCs, such as CD73, CD90, CD105 and are negative for CD14, CD34 and CD45.
In the pulp chamber, DPSCs are inactive, becoming active after injury. When dentin injury occurs, these cells migrate to the damaged region, proliferate and are able to differentiate towards osteoblast-like cells to form reparative dentin.16 In vitro, these cells have the capacity to differentiate into osteoblasts, adipocytes, chondroblasts, odontoblasts, muscle cells, neural cells, endothelial cells, hepatocytes, and melanocytes.17-23
•Stem Cells from Human Exfoliated Deciduous Teeth
SHEDs were first obtained by Miura et al.12 and are able to differentiate into adipocytes, chondroblasts, osteoblasts, odontoblasts, and muscle cells in vitro.1,24,25 In addition, they can also differentiate towards neural cell lines.1,24,25 In vivo, SHEDs do not differentiate directly into osteogenic cells but induce bone formation as well as assist in the process of angiogenesis.1,12
In comparison with DPSCs, SHEDs have higher cell proliferation rate, shorter population doubling time and increased clonogenic potential.25,26 Regarding the expression of surface markers, these two cell types are similar, being positive for mesenchymal markers and negative for hematopoietic markers.1,24
•Periodontal Ligament Stem Cells
The periodontal ligament is a connective tissue composed by, among other cell types, PDLSCs, which were first isolated by Seo et al.,13 from extracted human third molars.
PDLSCs exhibit self-renewal capacity and express cell surface markers similar to bone marrow-derived MSCs. They are able to differentiate towards osteoblasts, odontoblasts, adipocytes, neural cells, cementoblasts, and chondroblasts in vitro.1,13,20,27,28
•Stem Cells from Apical Papilla
SCAPs were initially isolated from third molars and incisors of swine by Sonoyama et al.29 and obtained from humans in 200815. SCAPs express STRO1, CD24, CD29, CD73, CD90, CD105, CD106, CD146, CD166, alkaline phosphatase (ALP) and do not express hematopoietic and endothelial markers (CD14, CD34 e CD45). Among these markers, CD24 would be specific of SCAPs, since it was not found in other DSCs.15,30 In addition, these cells also express neural markers.30
SCAPs differentiate towards osteoblasts, adipocytes, and odontoblasts in vitro, but chondrogenic differentiation potential has not been demonstrated.15,30 When associated with a hydroxyapatite scaffold and implanted in immunocompromised rats, formation of mineralized tissue (bone and dentin-like) was found.31 Moreover, these cells have significantly higher proliferation and mineralization potential in comparison with DPSCs.32
• Dental Follicle Stem Cells
Dental follicle is a loose connective tissue that surrounds the crown of non-erupted teeth.14 It originates the root cementum, periodontal ligament and alveolar bone during rhizogenesis, and coordinates the tooth eruption process.33
DFSCs were first obtained from human third molars by Yao et al.,14 who demonstrated the property of self-renewal, clonogenic potential and osteogenic differentiation. DFSC express mesenchymal surface markers and are negative for hematopoietic markers.1 These cells differentiate towards cementoblasts, adipocytes and cells of neural lineage in vitro.33-35 Few studies were able to perform chondrogenic differentiation.36,37 These studies suggest that DFSCs show variation in the ability to differentiate depending on the dental development stage. DFSCs also present heterogeneity in cellular proliferation rate.38
Discussion
DSCs are highly proliferative and multipotent cells, and can differentiate into many cell types. Therefore there is a growing interest in a better understanding of their potential and clinical applications. Moreover, the use of these cells in Medicine and Dentistry has been proposed because of their easy obtainment, being isolated from permanent teeth indicated for extraction or naturally exfoliated deciduous teeth. For these reasons, DSCs banks have been created around the world.39 In Brazil, The National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária – ANVISA) authorizes the storage of SCs originated from umbilical cord blood and placenta, as well as SHEDs in private establishments with proper operating license, according to the Resolution of Board Directors (Resolução da Diretoria Colegiada – RDC) 56, from 12/16/2010 and RDC 9, from 03/14/2011. Currently, private cell banks provide SCs collection and storage service, without promising treatment for any disease.
DSCs have been studied for the regeneration of various tissues and organs, such as bones, vascular system, liver, pancreas, and cornea.1,37Furthermore, these cells have also been studied for the treatment of neurodegenerative diseases.1,37 In the field of Dentistry, DSCs have been studied for the treatment of periodontitis, repair of maxillofacial bone problems, pulp regeneration after necrosis, as well as the development of new teeth.
• Use of the Dental Stem Cells for the Treatment of Periodontitis
Periodontitis is an inflammatory disease that causes injuries in the cementum, periodontal ligament and alveolar bone. Currently, there is no periodontal treatment that regenerates the modified region and the lost periodontal tissue into a normal and functional structure. As PDLSCs can differentiate into osteoblasts and cementoblasts, and induce tissue formation around the surface of dental implants, in vivo studies transplanted these cells associated with scaffolds in immunocompromised animal models and had showed regeneration of periodontal tissue.40,41 In humans, repair of structures affected by periodontal disease is possible.42,43 Feng et al.42 obtained significant improvement in the injured area after the use of PDLSCs, but only three patients were treated. In recent study, Chen et al.43 performed autologous transplant of PDLSCs and DPSCs in 30 patients and showed that the use of these cells in areas of periodontal disease is safe and the does not produce significant adverse effects. Thus, the use of DPSCs for the regeneration of bone loss from periodontal disease may be clinically relevant. Therefore, the use of different populations of DSCs in the treatment of periodontal disease can be an interesting approach.4
• Use of the Dental Stem Cells for Bone Regeneration
DPSCs have been studied not only for bone loss caused by periodontal disease, as already mentioned, but also for reconstruction of maxillofacial bones. D'Aquino et al.44 transplanted autologous DPSCs associated with a collagen scaffold to repair defects in alveolar bone secondary to extraction of impacted third molars in seven patients and, after three months, Scithere were bone regeneration areas. Three years after the transplant, Giuliani et al.45 showed that the transplanted area was made of uniformly vascularized compact bone, with bone matrix histologically different from the normal alveolar bone. Despite the histological difference, there was no difference in dental function and chewing.45 The authors suggest that the process of differentiation of CTPS can be more affected by the site of origin than by the signals sent by cells near the treated area.
• Use of the Dental Stem Cells for Pulp Regeneration
Dental pulp plays multiple roles in tooth homeostasis and maintenance of pulp tissue is important to its longevity.46 After endodontic treatment, the dental pulp is replaced with inorganic material, leaving the tooth devitalized and more likely to fracture. Thus, studies have been conducted seeking an effective strategy for pulp regeneration after endodontic treatment. 47 Currently, two strategies have been investigated: DPSCs autologous transplantation associated with scaffolds and pulp canal revascularization, which attract MSCs to the site of injury. However, histological analysis after the root canal and pulp chamber revascularization shows that there is no tissue formation similar to pulp.47 The majority of the cases present non-pulp-like tissues, comprising cementum, periodontal and bone-like-tissues.47 In a study in an animal model (dogs), pulp-like tissue with nerves and vasculature was regenerated in the tooth root after transplantation of a subset of DPSCs (CD105+) with stromal cell-derived factor-1 (SDF-1).46
• Use of the Dental Stem Cells for Formation of New Teeth
The development of a tooth is determined by several growth factors and complex interactions that result in tooth germ cell changes, leading to cell differentiation.1,4 Efforts have been employed to develop new teeth from DSCs. After subcutaneous transplantation of DPSCs and SHEDs in the dorsal surface of immunocompromised mice and rabbits, specialized dentin was produced and comprised of odontoblast-like cells surrounding an interstitial tissue similar to dental pulp.11,12,48 Seo et al.13 showed the ability of PDLSCs to form cementum in vivo after transplantation in the dorsum of immunocompromised mice. SCAPs actively participate in rhizogenesis and together with the PDLSCs can form root and periodontal ligament.1 Sonoyama et al.29 associated SCAPs and PDLSCs in a scaffold and showed the formation of structure similar to a tooth crown, capable of withstanding the impact resulting from mastication. Ikeda et al.49 had extracted the first molar from mice with five weeks of age and, after three weeks, transplanted the tooth germ stem cells in the alveolar bone located in the previously extracted tooth area. The resulting bioengineered product was a functional tooth with normal structure, with adequate response to pain and mechanical stress.49
Conclusion
Currently, the use of DSCs has been only applied in scientific research, but it is believed that, in a near future, this practice becomes a reality, which will represent a great advance in Dentistry.50 More studies on the DSCs differentiation mechanisms and applications are needed to use these cells in routine dental practice. The creation of public DSCs banks for research is an important step for this.
References
1. Har A, Park JC. Dental Stem Cells and Their Applications. Chin J Dent Res. 2015;18(4):207-12. [ Links ]
2. Ledesma-Martínez E, Mendoza-Núñez VM, Santiago-Osorio E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2016;2016:4709572.
3. Behr B, Ko SH, Wong VW, Gurtner GC, Longaker MT. Stem cells. Plast Reconstr Surg. 2010;126(4):1163-71.
4. Ashri NY, Ajlan SA, Aldahmash AM. Dental pulp stem cells. Biology and use for periodontal tissue engineering. Saudi Med J. 2015;36(12):1391- 9.
5. De Sá Silva F, Almeida PN, Rettore JVP, Maranduba CP, de Souza CM, de Souza GT, et al. Toward personalized cell therapies by using stem cells: seven relevant topics for safety and success in stem cell therapy. J Biomed Biotechnol. 2012;2012:758102.
6. Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381- 90.
7. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-7.
8. Cordeiro-Spinetti E, de Mello W, Trindade LS, Taub DD, Taichman RS, Balduino A. Human bone marrow mesenchymal progenitors: perspectives on an optimized in vitro manipulation. Front Cell Dev Biol. 2014;2:7.
9. Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells. 2014;6(5):526-39.
10. Potdar PD, Jethmalani YD. Human dental pulp stem cells: Applications in future regenerative medicine. World J Stem Cells. 2015;7(5):839-51.
11. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625-30.
12. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(25):5807-12.
13. Seo B-M, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149-55.
14. Yao S, Pan F, Prpic V, Wise GE. Differentiation of stem cells in the dental follicle. J Dent Res. 2008;87(8):767-71.
15. Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34(2):166-71.
16. Petrovic V, Stefanovic V. Dental tissue--new source for stem cells. SciWorld Journal. 2009;9:1167-77.
17. Alkhalil M, Smajilagić A, Redžić A. Human dental pulp mesenchymal stem cells isolation and osteoblast differentiation. Med Glas. 2015;12(1):27-32.
18. Koyama N, Okubo Y, Nakao K, Bessho K. Evaluation of pluripotency in human dental pulp cells. J Oral Maxillofac Surg. 2009;67(3):501-6.
19. Ahmed N, Aboul-Ezz E, Zakhary S, El Badry T, Ramzy M. Isolation of Dental Pulp Stem Cells and their In Vitro Differentiation into Odontoblast- like Cells. Maced J Med Sci. 2011;4(3):253-60.
20. Lee J-H, Um S, Song I-S, Kim HY, Seo BM. Neurogenic differentiation of human dental stem cells in vitro. J Korean Assoc Oral Maxillofac Surg. 2014;40(4):173-80.
21. Li X, Hou J, Wu B, Chen T, Luo A. Effects of platelet-rich plasma and cell coculture on angiogenesis in human dental pulp stem cells and endothelial progenitor cells. J Endod. 2014;40(11):1810-4.
22. Cho Y-A, Noh K, Jue S-S, Lee S-Y, Kim E-C. Melatonin promotes hepatic differentiation of human dental pulp stem cells: clinical implications for the prevention of liver fibrosis. J Pineal Res. 2015;58(1):127-35.
23. Paino F, Ricci G, De Rosa A, D'Aquino R, Laino L, Pirozzi G, et al. Ecto-mesenchymal stem cells from dental pulp are committed to differentiate into active melanocytes. Eur Cell Mater. 2010;20:295-305.
24. Annibali S, Cristalli MP, Tonoli F, Polimeni A. Stem cells derived from human exfoliated deciduous teeth: a narrative synthesis of literature. Eur Rev Med Pharmacol Sci. 2014;18(19):2863-81.
25. Eslaminejad MB, Vahabi S, Shariati M, Nazarian H. In vitro Growth and Characterization of Stem Cells from Human Dental Pulp of Deciduous Versus Permanent Teeth. J Dent Tehran. 2010;7(4):185-95.
26. Silva LB, Neto APDS, Pacheco RGP, Júnior SA, de Menezes RF, Carneiro VSM, et al. The Promising Applications of Stem Cells in the Oral Region: Literature Review. Open Dent J. 2016;10:227-35.
27. Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007;10(3):149-60.
28. Kim SS, Kwon D-W, Im I, Kim Y-D, Hwang D-S, Holliday LS, et al. Differentiation and characteristics of undifferentiated mesenchymal stem cells originating from adult premolar periodontal ligaments. Korean J Orthod. 2012;42(6):307-17.
29. Sonoyama W, Liu Y, Fang D, Yamaza T, Seo B-M, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. Plos One. 2006;1:e79.
30. Didilescu AC, Rusu MC, Nini G. Dental pulp as a stem cell reservoir. Romanian J Morphol Embryol. 2013;54(3):473-8.
31. Abe S, Yamaguchi S, Watanabe A, Hamada K, Amagasa T. Hard tissue regeneration capacity of apical pulp derived cells (APDCs) from human tooth with immature apex. Biochem Biophys Res Commun. 2008;371(1):90-3.
32. Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, et al. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol. 2011;56(7):709-21.
33. Guo W, Chen L, Gong K, Ding B, Duan Y, Jin Y. Heterogeneous dental follicle cells and the regeneration of complex periodontal tissues. Tissue Eng Part A. 2012;18(5-6):459-70.
34. Völlner F, Ernst W, Driemel O, Morsczeck C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differ. 2009;77(5):433-41.
35. Aonuma H, Ogura N, Takahashi K, Fujimoto Y, Iwai S, Hashimoto H, et al. Characteristics and osteogenic differentiation of stem/progenitor cells in the human dental follicle analyzed by gene expression profiling. Cell Tissue Res. 2012;350(2):317-31.
36. Kémoun P, Laurencin-Dalicieux S, Rue J, Farges J-C, Gennero I, Conte- Auriol F, et al. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007;329(2):283-94.
37. Park B-W, Kang E-J, Byun J-H, Son M-G, Kim H-J, Hah Y-S, et al. In vitro and in vivo osteogenesis of human mesenchymal stem cells derived from skin, bone marrow and dental follicle tissues. Differ. 2012;83(5):249-59.
38. Honda MJ, Imaizumi M, Tsuchiya S, Morsczeck C. Dental follicle stem cells and tissue engineering. J Oral Sci. 2010;52(4):541-52.
39. Gong T, Heng BC, Lo ECM, Zhang C. Current Advance and Future Prospects of Tissue Engineering Approach to Dentin/Pulp Regenerative Therapy. Stem Cells Int. 2016;2016:9204574.
40. Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z, et al. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells. 2010;28(10):1829-38.
41. Tsumanuma Y, Iwata T, Washio K, Yoshida T, Yamada A, Takagi R, et al. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials. 2011;32(25):5819-25.
42. Feng F, Akiyama K, Liu Y, Yamaza T, Wang T-M, Chen J-H, et al. Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis. 2010;16(1):20-8.
43. Chen F-M, Gao L-N, Tian B-M, Zhang X-Y, Zhang Y-J, Dong G-Y, et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33.
44. D' Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, et al. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75-83.
45. Giuliani A, Manescu A, Langer M, Rustichelli F, Desiderio V, Paino F, et al. Three years after transplants in human mandibles, histological and in-line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: biological and clinical implications. Stem Cells Transl Med. 2013;2(4):316-24.
46. Iohara K, Imabayashi K, Ishizaka R, Watanabe A, Nabekura J, et al. Complete Pulp Regeneration After Pulpectomy by Transplantation of CD105+ Stem Cells with Stromal Cell-Derived Factor-1. Tissue Eng Part A. 2011;17(15-16):1911-20.
47. Yang J, Yuan G, Chen Z. Pulp Regeneration: Current Approaches and Future Challenges. Front Physiol. 2016;7:58.
48. El-Backly RM, Massoud AG, El-Badry AM, Sherif RA, Marei MK. Regeneration of dentine/pulp-like tissue using a dental pulp stem cell/poly(- lactic-co-glycolic) acid scaffold construct in New Zealand white rabbits. Aust Endod J. 2008;34(2):52-67.
49. Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, et al. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci U S A. 2009;106(32):13475-80.
50. Feques R dos R, Freitas SAA, Pereira ALA, Vasconcelos A de F. Uso de células-tronco na Odontologia: realidade ou utopia? Braz J Periodonto 2014;24(03):24-30.
 Endereço para correspondência:
Endereço para correspondência:
Karin Soares Cunha
e-mail: karingcunha@gmail.com
Recebido: 20/09/2016
Aceito: 17/10/2016













