Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RPG. Revista de Pós-Graduação
versão impressa ISSN 0104-5695
RPG, Rev. pós-grad. vol.18 no.2 São Paulo Abr./Jun. 2011
ORIGINAL ARTICLE
Protein phosphatase activities in the serum and saliva of healthy children
Atividade de proteínas fosfatases no soro e saliva de crianças saudáveis
Antonio Hernandes Chaves NetoI; Kikue Takebayashi SassakiII; Ana Cláudia de Melo Stevanato NakmuneIII
IPhD in Biochemistry, Universidade of Campinas (UNICAMP); Substitute Teacher in the Discipline of Biochemistry – School of Dentistry, Universidade Estadual Paulista "Júlio de Mesquita Filho" de Araçatuba (UNESP) – Araçatuba/SP, Brazil.
IIPhD in Dentisty, University of São Paulo (USP) – São Paulo/SP; Assistant Professor in the Discipline of Biochemistry – School of Dentistry, Universidade Estadual Paulista "Júlio de Mesquita Filho" de Araçatuba (UNESP) – Araçatuba/SP, Brazil.
IIIPhD in Biochemistry, Universidade of Campinas (UNICAMP); Assistant Professor in the Discipline of Biochemistry – School of Dentistry, Universidade Estadual Paulista "Júlio de Mesquita Filho" de Araçatuba (UNESP) – Araçatuba/SP, Brazil.
ABSTRACT
The purpose of this study was to investigate the activities of the total acid phosphatase (TAP), tartrate-resistant acid phosphatase (TRAP), low molecular weight protein tyrosine phosphatase (LMW-PTP) and alkaline phosphatase (ALP) enzymes, as well as the possible correlation in the serum and in unstimulated whole saliva of children. Enzymatic activities were measured in pairs of concurrently obtained serum and salivary samples from 32 children in good oral and systemic health (16 of each sex) with a median age of 6.4 ± 3.3 years (range 1.08 – 12.92 years). All collections were made between the hours of 08:00 – 10:00 a.m. We used p-nitrophenyl phosphate as the substrate in the enzymatic assay for TAP, TRAP and LMW-PTP, and thymolphthalein monophosphate as the substrate for ALP. The enzymatic activities of all the studied enzymes were higher in serum than in saliva. The mean of enzymatic activities of serum TAP, TRAP, LMW-PTP and ALP were 36.51 ± 8.21, 23.99 ± 5.73, 11.16 ± 5.65 and 76.50 ± 17.32 U/L, respectively, while the mean salivary TAP, TRAP, LMW-PTP and ALP enzymatic activities were 9.60 ± 5.04, 1.36 ± 0.87, 5.65 ± 3.07 and 4.08 ± 1.83 U/L in this order. The TRAP revealed a positive linear correlation between its activity in the serum and saliva (Spearman r = 0,4685, p < 0,05). We concluded that the salivary TRAP has a potential to be use as biomarkers of pathologies and states that modify its activity in the serum.
Descriptors: Acid phosphatase. Alkaline phosphatase. Saliva. Serum.
RESUMO
O objetivo deste estudo foi investigar as atividades das enzimas fosfatase ácida total (FAT), fosfatase ácida resistente ao tartarato (TRAP), proteína tirosina fosfatase de baixa massa molar (LMW-PTP) e fosfatase alcalina (FAL), como também a possível correlação no soro e na saliva total não estimulada de crianças. As atividades enzimáticas foram mensuradas simultaneamente no soro e na saliva de 32 crianças (16 de cada sexo) com boa saúde oral e sistêmica, com média de idade de 6,4 ± 3,3 anos (variando entre 1,08 – 12,92 anos). Todas as coletas foram realizadas entre 8 e 10 h da manhã. Foi utilizado como substrato p-nitrofenil fosfato para a análise de FAT, TRAP e LMW-PTP, e timolftaleína monofosfato para análise de FAL. As atividades enzimáticas de todas as enzimas estudadas foram maiores no soro que na saliva. As médias ± dp das atividades enzimáticas para FAT, TRAP, LMW-PTP e FAL no soro foram 36,51 ± 8,21, 23,99 ± 5,73, 11,16 ± 5,65 e 76,50 ± 17,32 U/L, respectivamente, enquanto para a saliva foram, na mesma sequência, 9,60 ± 5,04, 1,36 ± 0,87, 5,65 ± 3,07 e 4,08 ± 1,83 U/L. A TRAP mostrou uma correlação linear positiva entre sua atividade no soro e na saliva (Spearman r = 0,4685, p < 0,05). Conclui-se que a TRAP salivar possui potencial para ser utilizada como biomarcador de patologias e estados que alterem sua atividade no soro.
Descritores: Fosfatase ácida. Fosfatase alcalina. Saliva. Soro.
INTRODUCTION
Analysis of saliva composition can be used as a diagnostic tool for the localization and evaluation of various oral and/or systemic pathologies, mainly when the correlation between the salivary and blood concentrations is high20,21. Easy storage and nontraumatic collection are an attractive aspect of saliva, especially to children, when repeated collections are required. The protein composition of saliva also reflects cellular signal processing that results from day-to-day environmental influences, as well as from acute or chronic stress9. Protein phosphatases reverse the covalent modifications of numerous cellular proteins imposed by the activation of protein kinases and, therefore, play key role in cell signaling metabolism, growth and differentiation. The activities of these enzymes have been evaluated in the saliva of children with alterations such as diabetes18 and cystic fibrosis22; nevertheless, few authors have compared the enzymatic activities of blood and saliva20,22.
Tartrate-resistant acid phosphatase (TRAP) belongs to a family of acid phosphatases that differ from the others isoforms of human acid phosphatase by being insensitive to tartrate and p-hydroxy mercury benzoate (p-HMB)17. Its most signifi cant expression occurs in osteoclasts, making this enzyme a marker of bone resorption12. Recent work showed that osteoblasts express TRAP activity in culture and this activity is modulated during osteoblastic differentiation5.
The protein tyrosine phosphatases (PTPs) are signaling enzymes involved in the regulation of numerous cell functions, including growth, mitogenesis, motility, cell-to-cell interactions, gene transcription and immune response15. In humans, class II cysteine-based PTPs are represented by the members of the low molecular weight protein tyrosine phosphatase (LMW-PTP) family. LMW-PTP expression is upregulated in various human cancers. Moreover, reports are found in literature showing the implications of deregulated activity and expression of LMW-PTPs in common diseases, including allergy, asthma, obesity, myocardial hypertrophy and Alzheimer´s disease2,8.
Human alkaline phosphatases (ALP) constitute multiple molecular forms of enzymes that are produced by many tissues, mainly by bone, liver, intestine and placenta, and are excreted by bile19. The seric dose of these enzymes is particularly useful in investigating bone diseases23. While elevated levels are found in bone diseases, characterized by the increase in osteoblastic function27, their levels are diminished in cases of chronic malnutrition1 and hypophosphatemia28.
The literature lacks studies analyzing TRAP and LMW-PTP activities in unstimulated whole saliva in children. These phosphatases are normally expressed in low concentrations, but the increase or significant reduction in their expression may occur as part of pathophysiological processes, enabling them to be used as serologic and histologic markers of diseases16,24.
OBJECTIVE
The purpose of this study was to evaluate the activities of the TAP, TRAP, LMW-PTP and ALP enzymes in serum and unstimulated whole saliva of children and the possible correlation between them.
MATERIAL AND METHODS
Thirty-two healthy children, aged 1 to 12 years, were submitted to clinical and oral exams, performed by a pediatrician and a pediatric dentist. Anamnesis was performed using the parents' reports and the children's health files. Only healthy, non medicated children, with good oral health were included in the study. The Research Protocol was approved by the Human Ethics Committee of the Faculdade de Odontologia de Araçatuba, Unesp – Universidade Estadual Paulista "Júlio de Mesquita Filho", Araçatuba/SP (Process Reference FOA 2003/558).
To minimize possible variations with reference to the circadian rhythm, the unstimulated whole saliva and blood were collected between 8:00 and 10:00 a.m., after 2 hours fast and oral hygiene with water, toothbrush and dental floss without fluoridated products. The salivary fluid present in the floor of the mouth was aspirated as described previously by Dezan et al.7 during 10 minutes. The saliva samples were centrifuged at 5.500 x g for 10 minutes, in a refrigerated centrifuge at 4ºC, to remove cellular and food debris, squamous cells and undissolved contaminants. The supernatants were fractionated and stored at – 70ºC for later analyses. Blood was collected immediately after saliva collection, by means of brachial vein puncture. The serum was obtained by centrifugation (1.500 x g) for 15 minutes, after 15 minutes rest at ambient temperature.
Total acid phosphatase (TAP), TRAP and LMW-PTP activities were determined using p-nitrophenyl phosphate (Sigma, St. Louis, USA) as the substrate as previously described5,6,10,11. ALP activity was assayed as the release of thymolphthalein from thymolphthalein monophosphate with a commercial kit-based method described by Roy26 (Labtest Diagnóstica, Lagoa Santa/MG, Brazil). One unit of enzymatic activity corresponds to the liberation of 1 μmol p-nitrophenol or 1 μmol thymolphthalein per minute. Enzymatic activity is expressed in units per litre (U/L).
The data were expressed as mean ± standard deviation of the mean. The data obtained presented a Gaussian distribution when submitted to the Kolmogorov-Smirnov (KS) test. The correlations among the enzymatic activities in whole saliva and in serum were analyzed by means of the Spearman's test. Statistical significance was assumed when p < 0.05. The statistical analyses were performed with the GraphPad Prism Version 3.00 software.
RESULTS
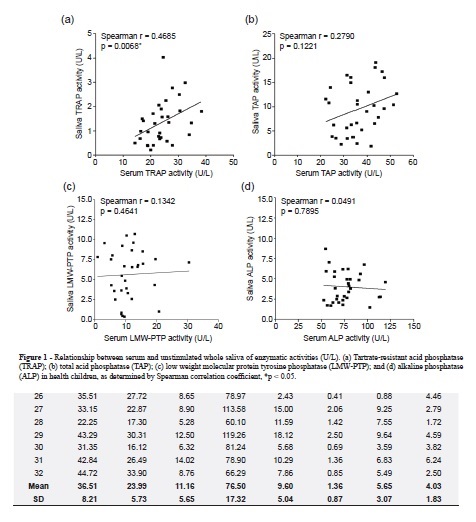
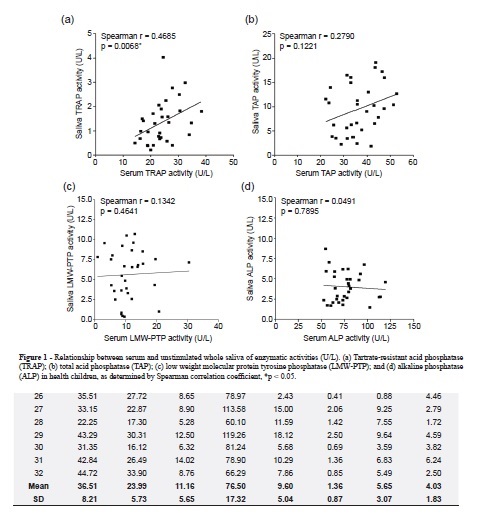
TAP, TRAP, LMW-PTP and ALP enzymatic activities (AE) were measured in pairs of concurrently obtained serum and salivary samples from 32 children with a median age of 6.4 ± 3.3 years (range 1.08 – 12.92 years). All enzymes analyzed were detected in both blood and saliva (Table 1). The enzymatic activities of all the studied enzymes were higher in serum than in saliva. The means ± sd of TAP, TRAP, LMW-PTP and ALP activities in serum were respectively: 36.51 ± 8.21, 23.99 ± 5.73, 11.16 ± 5.65 and 76.50 ± 17.32 U/L, and in saliva were: 9.60 ± 5.04, 1.36 ± 0.87, 5.65 ± 3.07 and 4.08 ± 1.83 U/L in the same sequence. Only scatter plot of the serum versus salivary TRAP enzymatic activities (Figure 1a) revealed a significant linear relationship (Spearman correlation coefficient, r = 0.4685, p = 0,0068). We have not observed any statistically significant linear relationship between serum versus salivary TAP (Figure 1b), LMW-PTP (Figure 1c) and ALP (Figure 1d) enzymatic activities.
DISCUSSION
The advantages of saliva in relation to serum as diagnostic fluid are easy handling, reduced blood-borne infection, non invasive method and subsequently more cooperation from children. This work is the first to investigate the activities of TRAP and LMW-PTP in saliva, and shows that there is a moderate positive correlation between serum and saliva TRAP activities.
Whole saliva is a complex mixture of secretion from the three paired major salivary glands (parotid, submandibular and sublingual) and from many minor salivary glands. In addition, it may contain gingival crevicular fluid, blood, leukocytes, desquamated cells, bacteria, dental plaque, virus and food debris. The presence and origin of certain salivary enzymes is still controversial. Nagler et al.20 described two fundamental processes that modulate saliva composition: first, the glands and oral sources may be the primary sites in which the various components are produced, or they may be merely the sites of passage through which various components are passively diffused or actively transported from the blood; second, various processes take place in the oral cavity after the secretion of saliva that alter its composition.
The demand to perform oral hygiene without the use of fluoridated dentifrice was made to prevent inhibition of the osteoclastic fraction of TRAP by fluoride13. To avoid the interference of brushing in the salivary protein concentration, related by Hoek et al.14, the samples were collected a minimum of two hours after the children performed oral hygiene. To minimize variations in salivary flow resulting from the dietary status, collections were made after the children had fasted for 2 hours.o24.
Activities of the all enzymes studied were detectable in both biological samples and were higher in serum than in saliva. The ratio of serum to salivary enzymatic activity for TAP, TRAP, LMW-PTP and ALP were 3.8, 17.66, 1.98 and 18.8, respectively. Nagler et al.20 also had described ALP activity in serum higher in comparison with those in saliva, however the enzymatic activity was only 5.73 times higher; on the other hand, in the same work, the TAP activity in saliva was 9.135 higher in comparison with those in serum. The existence of correlation between specific components in whole saliva and in blood is the point of origin to validate its use as biological material for clinical laboratory analysis; nevertheless, the absence of a full correlation between the concentrations of a component of saliva and of blood does not necessarily deny its blood origin20. In this work, only TRAP demonstrated correlation between its activities in the blood and whole saliva. This isoenzyme seems to be essential for demineralization of hard tissue in tooth roots and for digestion for demineralization of hard tissue3. Despite of our data, Nagler et al.20 showed the correlation between the activities of TAP and ALP in the blood/whole saliva. This divergence in results may be related to the different age groups (6.4 versus 30.8 years) and different substrates (p-nitrophenyl phosphate and thymolphthalein monophosphate vs 1-naphthylphosphate and p-nitrophenyl phosphate) used in this study.
Another important aspect to be considered is the differences between the TRAP and LMW-PTP activities in relation to proportion of TAP activities. The isoenzyme TRAP accounts for approximately 65.70% of the TAP activity in the serum and this proportion diminished in the saliva to 14.13%, whereas LMW-PTP comprises 30.57% of the TAP activity in serum and this proportion increased in the saliva to 58.90%. These data suggest that diffusion from the surrounding tissues through the salivary glands and the blood vessels may take place partially or processes like production of radical oxygen species occuring in the oral cavity could alter the enzymatic activity. It is known that the regulation of LMW-PTP activity can occur by a refined redox system due cysteine near the catalytic site and that hydrogen peroxide inactivates reversibly LMW-PTP4, while the regulation of TRAP activity occurs in a redox-dependent pathway, in which two ferric ions present in the enzyme determine its activity, being active when these ions are reduced and inactive when they are oxidized25. The enzymatic assay of saliva obtained separately from each pair of glands such as parotid, submaxillary/sublingual could explain the contribution of these glands in the secretion of TRAP e LMW-PTP and the influence of the chemical composition of whole saliva in the enzymatic activities.
CONCLUSION
The activities of all the examined enzymes (TAP, ALP, TRAP and LMW-PTP) were detected in the total unstimulated saliva and serum of normal children, being higher in serum than in saliva. However, we observed different proportions of TRAP and LMW-PTP activities in relation to TAP activities. It was observed a moderate correlation between serum and saliva TRAP activities.
Further investigations on saliva obtained separately from parotid and submaxillary/sublingual glands would provide new insights on the use of salivary enzymes as biochemical markers of diseases and saliva as diagnostic tool in children.
REFERENCES
1. Agarwal A, Gulati D. Early adolescent nutritional rickets. J Orthop Surg (Hong Kong) 2009;17(3):340-5. [ Links ]
2. Bottini N, Bottini E, Gloria-Bottini F, Mustelin T. Low-molecular-weight protein tyrosine phosphatase and human disease: in search of biochemical mechanisms. Arch Immunol Ther Exp (Warsz) 2002;50(2):95-104. [ Links ]
3. Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev 1996;17(4):333-68. [ Links ]
4. Caselli A, Marzocchini R, Camici G, Manao G, Moneti G, Pieraccini G, et al. The inactivation mechanism of low molecular weight phosphotyrosine–protein phosphatase by H2O2. J Biol Chem 1998;273(49):32554-60.
5. Malaspina TSS, Santos CX, Campanelli AP, Laurindo FR, Sogayar MC, Granjeiro JM. Tartrate-resistant acid phosphatase activity and glutathione levels are modulated during hFOB 1.19 osteoblastic differentiation. J Mol Histol 2008;39(6):627-34. [ Links ]
6. Malaspina TSS, Zambuzzi WF, Santos CX, Campanelli AP, Laurindo FR, Sogayar MC, et al. A possible mechanism of low molecular weight protein tyrosine phosphatase (LMW-PTP) activity modulation by glutathione action during human osteoblast differentiation. Arch Oral Biol 2009;54(7):642-50. [ Links ]
7. Dezan CC, Nicolau J, Souza DN, Walter LR. Flow rate, amylase activity, and protein and sialic acid concentrations of saliva from children aged 18, 30 and 42 months attending a baby clinic. Arch Oral Biol 2002;47(6):423-7. [ Links ]
8. Ferreira CV, Justo GZ, Souza AC, Queiroz KC, Zambuzzi WF, Aoyama H, et al. Natural compounds as a source of protein tyrosine phosphatase inhibitors: application to the rational design of small-molecule derivatives. Biochimie 2006;88:1859-73. [ Links ]
9. Granger DA, Kivlighan KT, el-Sheikh M, Gordis EB, Stroud LR. Salivary alpha-amylase in biobehavioral research: recent developments and applications. Ann N Y Acad Sci 2007;1098:122-44. [ Links ]
10. Granjeiro JM, Ferreira CV, Jucá MB, Taga EM, Aoyama H. Bovine kidney low molecular weight acid phosphatase: FMN-dependent kinetics. Biochem Mol Biol Int 1997;41:1201-8. [ Links ]
11. Granjeiro JM, Taga EM, Aoyama H. Purification and characterization of a low-molecular-weight bovine kidney acid phosphatase. An Acad Bras Cienc 1997;69(4):451-60. [ Links ]
12. Halleen JM, Tiitinen SL, Ylipahkala H, Fagerlund KM, Väänänen HK. Tartrate-resistant acid phosphatase 5b (TRACP 5b) as a marker of bone resorption. Clin Lab 2006;52(9-10):499-509. [ Links ]
13. Hayman AR, Cox TM. Purple acid phosphatase of the human macrophage and osteoclast. Characterization, molecular properties, and crystallization of the recombinant di-iron-oxo protein secreted by baculovirus-infected insect cells. J Biol Chem 1994;269(2):1294-300. [ Links ]
14. Hoek GH, Brand HS, Veerman EC, Amerongen AV. Toothbrushing affects the protein composition of whole saliva. Eur J Oral Sci 2002;110(6):480-1. [ Links ]
15. Huang JF. Different protein tyrosine phosphatase superfamilies resulting from different gene reading frames. Mol Biol Evol 2003;20(5):815-20. [ Links ]
16. Janckila AJ, Yam LT. Biology and clinical significance of tartrate-resistant acid phosphatases: new perspectives on an old enzyme. Calcif Tissue Int 2009;85(6):465-83. [ Links ]
17. Lau KH, Onishi T, Wergedal JE, Singer FR, Baylink DJ. Characterization and assay of tartrate-resistant acid phosphatase activity in serum: potential use to assess bone resorption. Clin Chem 1987;33(4):458-62. [ Links ]
18. López ME, Colloca ME, Páez RG, Schallmach JN, Koss MA, Chervonagura A. Salivary characteristics of diabetic children. Braz Dent J 2003;14(1):26-31. [ Links ]
19. Moss DW. Alkaline phosphatase isoenzymes. Clin Chem 1982;28:2007-16. [ Links ]
20. Nagler RM, Hershkovich O, Lischinsky S, Diamond E, Reznick AZ. Saliva analysis in the clinical setting: revisiting an underused diagnostic tool. J Investig Med 2002;50(3):214-25. [ Links ]
21. Nagler RM, Klein I, Zarzhevsky N, Drigues N, Reznick AZ. Characterization of the differentiated antioxidant profile of human saliva. Free Radic Biol Med 2002;32(3):268-77. [ Links ]
22. Oglesbee LH, Seale TW, Mayes JS, Flux M, Young SK, Renner OM. Plasma and submandibular saliva lysosomal enzymes in cystic fibrosis. Clin Chim Acta 1984;143(2):135-45. [ Links ]
23. Pagani F, Francucci CM, Moro L. Markers of bone turnover: biochemical and clinical perspectives. J Endocrinol Invest 2005;28(10 Suppl):8-13. [ Links ]
24. Pater A, Sypniewska G, Pilecki O. Biochemical markers of bone cell activity in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2010;23(1-2):81-6. [ Links ]
25. Räisänen SR, Alatalo SL, Ylipahkala H, Halleen JM, Cassady AI, Hume DA, et al. Macrophages overexpressing tartrate-resistant acid phosphatase show altered profile of free radical production and enhanced capacity of bacterial killing. Biochem Biophys Res Commun 2005;331(1):120-6. [ Links ]
26. Roy AV. Rapid method for determining alkaline phosphatase activity in serum with thymolphthalein monophosphate. Clin Chem 1970;16(5):431-6. [ Links ]
27. Singer FR. Paget disease: when to treat and when not to treat. Nat Rev Rheumatol 2009;5(9):483-9. [ Links ]
28. Whyte MP. Physiological role of alkaline phosphatase explored in hypophosphatasia. Ann N Y Acad Sci 2010;1192:190-200. [ Links ]
 Corresponding address:
Corresponding address:
Ana Cláudia de Melo Stevanato Nakamune
Departamento de Ciências Básicas da Faculdade de Odontologia da Universidade Estadual Paulista "Júlio de Mesquita Filho" de Araçatuba (UNESP)
CEP 16018-805 – Araçatuba/SP, Brazil
Phone: (18) 3636-2788 / Fax: (18) 3636-5267
e-mail: anacmsn@foa.unesp.br
Received in: 24/2/11
Accepted in: 5/5/11













