Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RPG. Revista de Pós-Graduação
versão impressa ISSN 0104-5695
RPG, Rev. pós-grad. vol.19 no.4 São Paulo Out./Dez. 2012
ORIGINAL ARTICLE
Influence of dehydration on strain pattern of human and bovine teeth: in vitro study
Influência da desidratação no padrão de deformação de dentes humanos e bovinos: estudo in vitro
PAULO VINÍCIUS SOARESI; FABRÍCIA ARAÚJO PEREIRAI; BRUNO RODRIGUES REISII; LIVIA FAVÁRO ZEOLAI; CARLOS JOSÉ SOARESI; MURILO SOUZA MENEZESI
I Dental School, Universidade Federal de Uberlândia (UFU) – Uberlândia/MG, Brazil
II Dental School, Universidade de São Paulo (USP) – Sao Paulo/SP, Brazil
ABSTRACT
The aim of this study was to measure the effect of the elapsed time on micro-strain of dental structure by strain gauge test of human and bovine teeth. Ten standardized bovine incisors and ten standardized human premolars were obtained. To measure the strain it was fixed one strain gauge on the buccal of each sample and it was also performed a compression test on each of dehydration times: T0: immediately after removal from moisture; T5: after dehydration in room temperature (23 ± 1oC) for 5 minutes; T15: dehydration for 15 minutes; T45: dehydration for 45 minutes; T120: dehydration for 120 minutes; T24: dehydration for 24 hours; T24Re: dehydration for 24 hours and rehydration for 24 hours in distilled water. The results were statistically analyzed by using one-way ANOVA and Tukey’s test (p < 0.05). Mean and standard deviation values in micro-Strain (μS) of human teeth were: T0: 56.7 ± 28.5 a; T5: 56.9 ± 43.7 a; T15: 40.2 ± 29.7 ab; T45: 41.2 ± 22.1 ab; T120: 32.2 ± 22.5 ab; T24: 22.6 ± 21.7 b; T24Re: 38.5 ± 12.9 ab, and bovine teeth were: T0: 47.5 ± 15.6 a; T5: 39.2 ± 12.5ab; T15: 34.2 ± 11.5 abc; T45: 29.0 ± 7.9 bc; T120: 25.3 ± 10.5 bc; T24: 22.6 ± 10.2 bc; T24Re: 29.9 ± 13.2 bc. The μS values were decreased with increasing of the dehydration time for both teeth groups. It can be concluded that dehydration caused strain decreasing being necessary to keep the samples hydrated during strain gauge tests.
Descriptors: Dental Enamel. Dentistry. Bicuspid.
RESUMO
O objetivo deste estudo foi medir o efeito do tempo decorrido em microdeformação da estrutura dental por teste de extensometria de dentes humanos e bovinos. Obtiveram-se dez incisivos bovinos padronizados e dez pré-molares humanos padronizados. Para medir a deformação foram fixados extensômetros sobre a face vestibular de cada amostra, e também foi realizado um teste de compressão em cada um dos tempos de desidratação: T0: imediatamente após a remoção da umidade; T5: após desidratação em temperatura ambiente (23 ± 1°C) por 5 minutos; T15: desidratação por 15 minutos; T45: desidratação durante 45 minutos; T120: desidratação por 120 minutos; T24: desidratação por 24 horas; T24Re: desidratação por 24 horas e re-hidratação durante 24 horas em água destilada. Os resultados foram analisados estatisticamente utilizando o one-way ANOVA e teste de Tukey (p < 0,05). Os valores da média e desvio-padrão em microdeformação (μS) de dentes humanos foram: T0: 56,7 ± 28,5 a; T5: 56,9 ± 43,7 a; T15: 40,2 ± 29,7 ab; T45: 41,2 ± 22,1 ab; T120: 32,2 ± 22,5 ab; T24: 22,6 ± 21,7 b; T24Re: 38,5 ± 12,9 ab e dentes bovinos foram: T0: 47,5 ± 15,6 a; T5: 39,2 ± 12,5 ab; T15: 34,2 ± 11,5 abc; T45: 29,0 ± 7,9 bc; T120: 25,3 ± 10,5 bc; T24: 22,6 ± 10,2 bc; T24Re: 29,9 ± 13,2 bc. Os valores μS foram reduzidos com o aumento do tempo de desidratação para ambos os grupos de dentes. Pôde-se concluir que a desidratação causada promoveu maior rigidez da estrutura, sendo necessário manter as amostras hidratadas durante testes laboratoriais.
Descritores: Esmalte dentário. Odontologia. Dente premolar.
INTRODUCTION
The primary function of the dental element is the transmission of muscular force during the process of preparation of food24. A compound harmoniously constituted by the structure of mutual protection between enamel and dentin establishes a stable state of stressstrain during occlusal forces5,20. Enamel is the most mineralized dental tissue, which contains 96% mineral, 1% organic material, 3% water. The composition of this structure is approximately 89% inorganic components, 2% organic components and 9% water5. Dentin is considered more permeable than the enamel due to its higher organic content and tubular structure. Dentin weight is composed approximately by 70% minerals, 18% organic material and 12% water and its volume shows 45% minerals, 30% organic matter and 25% water12,16. Small modifications in the structural composition of the dentin and enamel can modify the structural compound functions as to its capacity of receiving masticatory forces1. Enamel is the dental structure specifically formed to support the chemical, thermal and physical variations of the oral cavity. Most free water within enamel is located in the protein matrix and has an influence on the enamel capacity of compressibility, in permeability and ionic conductivity6. The change of concentration or composition of these structures can influence directly in the mechanical properties of the enamel6. Several studies have employed the association of different experimental tests, as fracture resistance6,18-20, knoop hardness and nanoindentation tests6, and strain gauge test23 to analyze the mechanical behavior of the dental structure. For strain analysis of dental structure several experimental methods and computer-aided tests have been used. The strain gauge test constitutes a non-destructive test for measuring external strain of the dental structure. This test makes possible the analysis and through mathematical calculations it is possible to calculate the structure strain. Due to evolution of the restoring techniques and mainly preventive dentistry, the use of human teeth in laboratory research has become limited for the greater maintenance of teeth in the oral cavity. Nowadays, human teeth have being substituted by bovine teeth, which are more easily collected, making possible the standardization of the age, as well as minimizing risk of transmission of infectious diseases4,13. There are some instances in which routine clinical practices can cause changes in the hydration of teeth tissues, like evaluation of pulpal sensitivity using forced air, rubber-dam procedures for long time, and during enamel/dentin preparation for bonding procedure. The water loss imposed by these procedures can result in dentin and enamel strain, if restrained, it can cause the development of undesired and potentially deleterious stress2. Therefore, the aim of this study was to measure the influence of dehydration on the superficial strain of dental structure, testing the hypothesis that the enamel and dentin dehydration reduce the capacity of dental structure strain.
MATERIALS AND METHODS
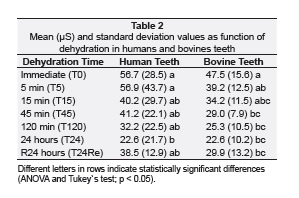
Ten bovine central incisors of adult animals, with the same size and age were collected, and ten intact mandibular human premolar teeth with similar coronary dimensions (Approved by Ethic Committee at Universidade Federal de Uberlândia, Protocol number 051/09), were collected. The teeth were cleaned with periodontal curette and submitted to prophylaxis with stone pumice and water, and stored in distilled water under 4°C. Teeth were embedded into polystyrene resin cylinder. Root surface was covered with cyanoacrylate glue (Super Bonder, Loctite) to prevent dehydration thought the root surface. To measure dental strain,one strain gauge (PA- 06-060BG-350L; Excel Sensores, Sao Paulo, Brazil) was fixed parallel to the long axis of each specimen, positioned on the center of the buccal face of bovine incisor or premolar parallel to the long axis of the tooth, positioned at 25 from the load application axis23. According to the manufacturer (Excel Sensores), the base material of these gauges consisted of a polyimide and metal constantan film, with temperature self-compensation for steel. The strain gauge grid had an area of 4.1 mm2 and an electrical resistance of 350 Ω22. Strain gauges used for this study had a gauge factor of 2.12 that is a proportional constant between electrical resistance variation and strain. To fix strain gauge, a 37% phosphoric acid solution (3M ESPE, St. Paul, Minnesota, USA) was applied for 15 seconds to each enamel surface, which were then washed with water for 15 seconds and dried with air spray. The strain gauges were bonded on cervical region of human and bovine enamel with cyanoacrylate adhesive (SuperBonder; Loctite, Sao Paulo, Brazil), and the wires were connected to the data acquisition device (ADS0500IP; Lynx Tecnologia Eletrônica Ltda, Sao Paulo, Brazil). In addition, strain gauge was fixed to another specimen, which no load was applied, to compensate for dimensional alterations due to temperature. These samples were important for the measurement of strain, without the influence of environmental temperature (Figure 1). The samples were submitted to axial compression loading at a speed of 0.5 mm/min, applied with a wedge-load device shape (Figure 1), until 250 N in a mechanical testing machine (EMIC DL 2000; EMIC Equipamentos e Sistemas de Ensaio Ltda, Sao José dos Pinhais, Brazil). The data were transferred to a computer that used specific acquisition, signal transformation and data analysis software (AqDados 7.02 and AqAnalisys; Lynx Tecnologia Eletrônica Ltda). During load application, the data acquirer collected one strain value every 0.3 seconds until a maximum load of 250 N was attained (Figure 1). The strain measurement results (μS) were obtained by values for the buccal cusp. The strain (μS) of all teeth of each group were analyzed sequentially in 6 moments (n = 10) according to Table 1.
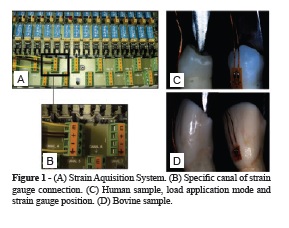
Data were recorded and analyzed using a oneway ANOVA followed by Tukey HSD test for each teeth group. One-way analysis of variance and Tukey’s method were performed with the use of a statistical software package (SPSS 15.0J for Windows; SPSS Japan, Tokyo, Japan), p < 0.05 was considered to be statistically significant.
RESULTS
One-way ANOVA showed significant differences. The mean (μS) and standard deviation (SD) values for all groups are showed on Table 2. For human teeth, samples dehydrated showed a decrease in the values of strain, so the more dehydrated the tooth is, the less is its ability to deform when subjected to a load. The statistically difference is showed in the Tukey’s test (Table 2) (p < 0.05), which revealed that T0 and T5 showed significantly higher strain values than T24 (Table 2). There was not statistically difference between T0 and T24Re, showing that re-hydration for 24 hours recovers the strain levels similar to hydrated samples. Bovine dehydrated teeth demonstrated significantly decreasing for strain values. The T0 samples showed statistically higher strain values than T24.
DISCUSSION
The hypothesis of this study was accepted that dehydration induced there decreasing of capacity of dental structure strain. The structure sound teeth loss promoted by fractures21, carie15, cavity preparations1 and endodontic treatment15,21 may cause changes the biomechanical behavior of the teeth. However, little has been studied about the reduction of water that is an important component of sound teeth. The water plays an important role not only in the dentin10, but in the enamel as well1, Mature dentine contains approximately 50% of inorganic phase and 40% of organic one. The major part of the organic phase is collagen and polymer that normally exist in an aqueous environment8,11. It is often associated with proteoglycans which contain a large amount of bound water. The inorganic or mineral phase in dentine is mostly a carbonated apatite11. Mature dentine contains approximately 10% water by weight and 22% water by volume8. It has been calculated that 75.2% water is in the dentinal tubules, and 24.8% is in the mineralized matrix. Mature enamel contains 94‒96% hydroxyapatite crystallites, with the remainder being mainly organic matrix and water5. As it is well known, hydroxyapatite is thermally and chemically stable under the above experimental conditions. Therefore, most of the mechanical response performed on different environments must be associated with the changing of properties of the organic matrix and water loss5. Water acts in the inter-connection of mineral and protein structure, its loss can end up in severe alterations in the biomechanical behavior of the dental structure. Therefore, the absence of water can lead to structural defects causing different behavior both at the time of biomechanical testing and clinically9. Free water in dentine is a dynamic phase, and that movement through the porosities and tubules in dentine may influence the response of bulk dentine structure during mechanical functions. Previous studies have suggested that the fluid filled dentinal tubules could function by hydraulically transferring and dissipating the occlusal forces applied to teeth7,14. Several studies8,9,11 have analyzed the influence of the loss of water on the biomechanical behavior of dental structure. And they concluded that the loss water in dentine induces a lower capability of strain and stress at fracture when compared to fully hydrated dentine samples. And the magnitude of elastic or reversible strains experienced by fully hydrated dentine was conspicuously higher than the partially dehydrated dentine. This study agrees with a recent analysis, so it is possible to observe that the loss of water decreases the strain capacity (Table 1). Complete hydration, i.e., presence of free water in the tubulo-canalicular system increases both resilience and toughness of the dentine. The increasing of the resilience (the capacity of a material to absorb energy elastically upon loading) and toughness (the ability to resist fracture) imply that the free water in the tubulo-canalicular systems provides dentine the ability to absorb loads elastically and consequently increase the fracture resistance. Study of the biomechanical behavior of fully hydrated and dehydrated dentine using moiré interfermometry, demonstrated that the presence of water resulted in a stress/strain response characteristic of tough material, while the loss of free water resulted in stiffening, and response characteristic of brittle material9. Recent studies demonstrated that the anisotropy of the enamel is dependent on another structure as important as the prism. Proteins and water present in the prismatic interfaces act as agents of interprismatic union6. Thus, the behavior of the structure of the enamel undergoes major change occurs when water loss, and in general we can conclude that both dentin and enamel for normal anisotropic characteristics in tooth structure would be jeopardized. This fact induces a changed biomechanical behavior as can be seen the difference in pattern of strain as the samples suffered dehydration process. There is a straight downward by decreasing the values of superficial strain in long axis. Enamel and dentin from different animal species have been for long used as substitutes for human ones, since mammalian and human teeth are morphohistologically similar to each other. The constant use of bovine teeth in dental researches has stimulated a number of studies searching to certify the suitability of this procedure. Dental hard tissues characteristics are the principal factor when analyzing the possibilities of substituting the usage of human teeth by animal teeth on in vitro researches. If there were no significant differences between human and animal teeth, then the results of any research performed with these animal teeth would have more value and significance to the dental community3,13. But as there are some differences, human enamel presented less interprismatic substance and distinct prisms, and bovine enamel showed greater amount of interprismatic substance with ‘‘fibril-like’’ structures3. Bovine dentin showed a relative visual lower presence of intertubular dentin than human one, together with a relatively more presence of peritubular dentin3. Thus, bovine teeth have a different behavior as can be observed through the results of this study (Table 2). However, in this study, the comparison between groups of teeth was not done, mainly for the fact that teeth used were not identical, to bovine incisors used, which have a coronal portion larger and therefore more structure, and loss of water slower than in human teeth. The premolars have a lower crown portion, and thus a smaller amount of water to be lost. The use of non-destructive tests gives information on the structural behavior of complex dental, which is an experimental method for measuring different types of strain through the use of gauges adhered on the external face of the dental structure17. The application of a load generates a stress that result in structural strain, and may increase according to the geometry and mechanical properties, exceeding the elastic regime until the collapse of the structure23, which is non-destructive. In this study, the method of strain gauge test enabled structural changes was observed in the microstrain pattern of the samples the extent to which they were dehydrated. The gauge adhered to the outside surface of the tooth, measured the performance of the overall tooth structure before the application of a load. The results agree with studies of whose found that dehydration effect on mechanical characteristics samples6,9.
CONCLUSION
Within the limitations of the present study it was possible to conclude that the free water influenced stress-strain on the distribution of the occlusal forces applied to teeth. And the loss water promoted a modification in the dental structure; thus the necessary care in research laboratory with the samples, stages of preparation of laboratory samples, moldings, cavity preparations and during the testing, and these always be hydrated. Dehydration of the enamel and dentin should have special attention, as can be observed through this study that the loss of water for the external middle due to dehydration of enamel and dentin reduces the capacity of dental structure strain.
ACKNOWLEDGEMENTS
The authors thank Fundação de Amparo à Pesquisa do estado de Minas Gerais (FAPEMIG), for financial support (#D30/2009).
BIBLIOGRAPHIC REFERENCES
1. Baldassarri M, Margolis HC, Beniash E. Compositional determinants of mechanical properties of enamel. J Dent Res 2008;87(7):645-9. [ Links ] 2. Castrisos TV, Palamara JE, Abbott PV. Measurement of strain on tooth roots during post removal with the Eggler post remover. Int Endod J 2002;35(4):337-44. [ Links ] 3. Fonseca RB, Haiter-Neto F, Carlo HL, Soares CJ, Sinhoreti MA, Puppin-Rontani RM, et al. Radiodensity and hardness of enamel and dentin of human and bovine teeth, varying bovine teeth age. Arch Oral Biol 2008;53(11):1023-9. [ Links ] 4. Fonseca RB, Haiter-Neto F, Fernandes-Neto AJ, Barbosa GA, Soares CJ. Radiodensity of enamel and dentin of human, bovine and swine teeth. Arch Oral Biol 2004;49(11):919-22. [ Links ] 5. Gwinnett AJ. Structure and composition of enamel. OperDent 1992;(Suppl)5:10-7. [ Links ] 6. He LH, Swain MV. Influence of environment on the mechanical behaviour of mature human enamel. Biomaterials 2007;28(30):4512-4520. [ Links ]
7. Hirata K, Nakashima M, Sekine I, Mukouyama Y, Kimura K. Dentinal fluid movement associated with loading of restorations. J Dent Res 1991;70(6):975-8. [ Links ] 8. Kinney JH, Balooch M, Marshall GW, Marshall SJ. Atomic-force microscopic study of dimensional changes in human dentine during drying. Arch Oral Biol 1993; 38(11):1003-7. [ Links ] 9. Kishen A, Asundi A. Experimental investigation on the role of water in the mechanical behavior of structural dentine. J Biomed Mater Res A 2005;73(2):192-200. [ Links ] 10. Kishen A, Vedantam S. Hydromechanics in dentine: role of dentinal tubules and hydrostatic pressure on mechanical stress-strain distribution. Dent Mater 2007;23(10):1296-306. [ Links ] 11. Marshall GW Jr. Dentin: microstructure and characterization. Quintessence Int 1993;24(9):606-17. [ Links ] 12. Mjör IA. Human coronal dentine: structure and reactions. Oral Surg Oral Med Oral Pathol 1972;33(5):810-23. [ Links ] 13. Nakamichi I, Iwaku M, Fusayama T. Bovine teeth as possible substitutes in the adhesion test. J Dent Res1983;62(10): 1076-81. [ Links ]
14. Pashley DH. Dentine permeability: Theory and practice. In: Spangberg LSW, editor. Experimental endodontics. Boca Raton, FL. CRC Press Inc 1990. p. 19-49. [ Links ] 15. Reeh ES, Messer HH, Douglas WH. Reduction in tooth stiffness as a result of endodontic and restorative procedures. J Endod 1989;15(11):512-6. [ Links ] 16. Rizzutto MA, Tabacniks MH, Added N, Liguori Neto R, Acquadro JC, Vilela MM, et al. External PIGE-PIXE measurements at the São Paulo 8UD tandem accelerator. Nucl Instr Meth Phys Res B 2002;190:186-9. [ Links ] 17. Sakaguchi RL, Brust EW, Cross M, DeLong R, Douglas WH. Independent movement of cusps during occlusal loading. Dent Mater 1991;7(3):186-90. [ Links ] 18. Sathorn C, Palamara JE, Palamara D, Messer HH. Effect of root canal size and external root surface morphology on fracture susceptibility and pattern: a finite element analysis. J Endod 2005;31(4):288-92. [ Links ] 19. Soares CJ, Martins LR, Fonseca RB, Correr-Sobrinho L, Fernandes Neto AJ. Influence of cavity preparation design on fracture resistance of posterior Leucite-reinforced ceramic restorations. J Prosthet Dent 2006;95(6):421-9. [ Links ] 20. Soares CJ, Soares PV, Santos-Filho, PCF, Castro, CG, Versluis A. The influence of cavity design and glass fiber posts on biomechanical behavior of endodontically treated premolars. J Endod 2008;34(8):1015-9. [ Links ] 21. Soares PV, Santos-Filho PC, Gomide HA, Araujo CA, Martins LR, Soares CJ. Influence of restorative technique on the biomechanical behavior of endodontically treated maxillary premolars. Part II: strain measurement and stress distribution. J Prosthet Dent 2008;99(2):114-22. [ Links ] 22. Soares PV, Santos-Filho PC, Martins LR, Soares CJ. Influence of restorative technique on the biomechanical behavior of endodontically treated maxillary premolars. Part I: fracture resistance and fracture mode. J Prosthet Dent 2008; 99(1):30-7. [ Links ] 23. Soares PV, Santos-Filho PC, Queiroz EC, Araújo TC, Campos RE, Araújo CA, et al. Fracture resistance and stress distribution in endodontically treated maxillary premolars restored with composite resin. J Prosthodont 2008;17(2):114-9. [ Links ] 24. Tantbirojn D, Feigal RJ, Ko CC, Versluis A. Remineralized dentin lesions induced by glass ionomerdemonstrate increased resistance to subsequent acid challenge. Quintessence Int 2006;37(4):273-81. [ Links ]
 Endereço para correspondência:
Endereço para correspondência:
Paulo Vinicius Soares
LCNC Research Group – School of Dentistry – Department of Operative Dentistry and Dental Materials ‒ Biomechanic Research Group – Universidade Federal de Uberlândia
Avenida Pará,1720, Bl. 2B, Sl. 24,
Zip Code 38400-902 – Uberlândia/MG, Brazil
e-mail: paulovsoares@yahoo.com.br
Received on: 8/6/12
Accepted on: 11/29/12













