Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RFO UPF
versão impressa ISSN 1413-4012
RFO UPF vol.16 no.2 Passo Fundo Mai./Ago. 2011
Preliminary histological analysis of methotrexate-induced oral mucositis: experimental study in mice
Análise histológica da mucosite bucal induzida por metotrexato: estudo piloto experimental em camundongos
Jéssica Cerioli Munaretto*; Deise Ponzoni**; Luiz Aida Sabbagh-Haddad***; Edela Puricelli****
* DDS, MSc, Specialist in Dentistry for Patients with Special Needs, Specialization Course, ABENO, SP, Brazil.
** DDS, MSc, PhD, Associate Professor, Department of Surgery and Orthopedics, Faculty of Dentistry, Federal University of Rio Grande do Sul, Porto Alegre, RS, Brazil.
*** DDS, MSc, PhD, Professor and Coordinator, Area of Dentistry for Patients with Special Needs, Specialization Course, ABENO, SP, Brazil.
**** DDS, MSc, PhD, Professor and Coordinator, Area of Oral and Maxillofacial Surgery and Traumatology, Post-Graduation Program in Dental Clinic, Faculty of Dentistry, Federal University of Rio Grande do Sul, Porto Alegre, RS, Brazil. Head of the Oral and Maxillofacial Surgery and Traumatology Service, Hospital de Clínicas de Porto Alegre, Porto Alegre, RS, Brazil.
Abstract
Objective: This in vivo experimental study evaluated the effect of methotrexate on the oral mucosa of mice. Methodology: A total of 28 adult BALB/c mice were randomly assigned into a control and an experimental group. Mice in the experimental group were immunosuppressed with 2.5 mg/kg of methotrexate for three consecutive days. One mouse from each group was sacrificed each day for 10 days. Samples from ventral mucosal tongue surface were collected and prepared for histomorphometric analysis, by staining with hematoxylin/ eosin. Three microscopic fields per section were analyzed, in order to evaluate the mean epithelial cells layer thickness and counting of blood vessels. To evaluate the existence of inflammatory infiltration, values of 0 and 1 were used for absence or presence, respectively. Results: The results showed that the treatment did not induce clinical signs of mucositis in the ventral mucosal tongue region. The histological examination showed that the mean number of blood vessels was similar in control and experimental samples (p < 0.001), and that there was absence of inflammatory infiltration. However, the thickness of the epithelial cell layer of experimental samples was significantly lower than in control samples. Conclusion: This study showed that, in the mouse model, methotrexate-induced mucositis histological result in manifestations in the ventral tongue region.
Key words: Methotrexate. Mucositis. Histology. Mice.
Resumo
Objetivo: Este estudo experimental in vivo se propôs avaliar o efeito do metotrexato sobre a mucosa bucal de camundongos. Metodologia: Foram utilizados 28 camundongos Mus musculus, Balb/c, selecionados de forma randomizada e divididos em grupo teste e grupo de controle. Os camundongos do grupo teste foram imunossuprimidos com 2,5 mg/kg de metotrexato durante três dias consecutivos. Um camundongo de cada grupo foi sacrificado diariamente, durante dez dias. Para este estudo piloto foi analisada a superfície ventral lingual de cada camundongo. Para a análise morfométrica-hematoxilina/ eosina foram capturados três campos microscópicos por lâmina do ventre lingual que apresentaram menor espessura epitelial. Para a avaliação da presença de infiltrado inflamatório convencionou-se 0 para ausência e 1 para presença de infiltrado inflamatório. A contagem dos vasos sanguíneos foi realizada por campo e foram registrados os vasos que apresentavam hemácias no seu interior. Resultados: Os resultados mostraram que não houve manifestações clínicas da mucosite na região de ventre lingual no grupo teste. Na análise histológica não houve nenhuma diferença estatística quanto à quantidade média de vasos (p < 0,001) e em ambos grupos, teste e controle, não foram observadas presença de infiltrado inflamatório. Entretanto, a espessura epitelial do grupo teste mostrou resultados significativamente menores em comparação ao grupo de controle. Conclusão: Este estudo mostrou que o metotrexato induziu resultado histológico de mucosite na superfície ventral da língua dos camundongos.
Palavras-chave: Metotrexato. Mucosite. Histologia. Camundongo.
Introduction
At the beginning of last century, the only treatment available for patients with solid tumors was surgery, associated with high morbidity and mortality. Over the past 40 years, chemotherapy resulted in a progressive improvement in survival rates of patients with malignant neoplasms1.
The drugs used in chemotherapy destroy rapidly dividing cells in the body or slow cell division nonspecifically. Similar to radiotherapy, normal host cells with a high index of mitotic activity are also affected. The cells most affected are from the gastrointestinal epithelium (including the epithelium of the buccal cavity) and bone marrow cells1.
The first experimental studies of chemotherapyinduced oral mucositis investigated the effect of the antimetabolite 5-fluorouracil on hamsters2. Since then, other studies have been conducted in rats and mice, with different inducing agents3-8. Mota in 20046, for instance, used methotrexate to induce intestinal mucositis in rats, based on the experimental model of Vanderhoof et al.9 (1990). Methotrexate is an antineoplastic, antipsoriatic, antirheumatic drug with immunosuppressive activity. This effect is observed for instance in mice treated subcutaneously with doses of 2.5 mg/kg during three consecutive days6,9-11.
Mucositis is an inflammatory, painful and debilitating condition that significantly interferes with anticancer therapy, particularly in patients undergoing radiotherapy and/or chemotherapy for head and neck cancer. Sequelae include severe pain, impairment of oral and pharyngeal function, bleeding episodes and increased risk for local and systemic infections2,12-15. The mucosa of the lips, mouth, tongue, floor of mouth and soft palate are more frequently affected by chemotherapy than keratinized hard tissues, such as the hard palate16.
According to World Health Organization (WHO), mucositis can be classified clinically into categories 0 to 4, as follows: 0, no mucosal lesion; 1, erythematous area; 2, mucosal erythema or ulceration, allowing intake of solid food; 3, ulcerated area, only liquids allowed; and 4, mucositis requiring enteral or parenteral nutrition17,18. Histologically, the disease proceeds in four phases: inflammatory/vascular, epithelial, ulcerative/bacterial, and healing phase. These stages are interdependent and result from cytokine-mediated effects of chemotherapy drugs on the epithelium and the composition of the oral microbiota2.
This study aimed to evaluate the effects of methotrexate treatment on the tongue mucosa in a murine model of oral mucositis. Parameters such as epithelial thickness of the cell layer, presence of inflammatory infiltrate and vascularization of the ventral tongue were compared in treated and control animals, in different periods.
Materials and method
In this in vivo experimental study, 2-month-old male BALB/c mice with an average weight of 30 g were used. The animals were kept under standard conditions (12 hours light/12 hours dark, water and food ad libitum). A total of 28 animals were randomly assigned to two groups. Mice in the experimental group (EG) were immunosuppressed with subcutaneous injections of 2.5 mg/kg methotrexate into the dorsal skin, for three consecutive days. The total dose corresponded to 0.3 mg. Mice in the control group (CG) were not treated. During each of the next ten days, one mouse from each group was killed by cervical dislocation19-21. Regions of the jugal mucosa, both submandibular glands, hard palate, esophagus and tongue were thoroughly collected and stored for later investigation. Mucosal tissue integrity and the presence or absence of mucositis were evaluated by macroscopic analysis. Clinical observations were classified according to the scale of the World Health Organization18. To avoid the post-mortem changes, the material was preserved in formalin 10% for a period of 48 hours.
The present study aimed to analyze the ventral lingual mucosal surfaces of treated and nontreated mice. Three-micrometer-thick sections were obtained from paraffin- embedded samples using a microtome (Leica, Germany) and stained with hematoxylin and eosin (HE), for analysis under light microscopy.
For morphometric and histological analyses, three microscopic fields from each ventral tongue mucosal epithelium section showing less ephetelial cell layers were registered. Analyses were performed with magnification of 400X, using a video camera (Olympus®, QColor 5, Coolet, RTV] attached to a binocular microscope (Olympus Optical Co.®, CX41RF] and a computer (Dell, Dimension 5150), using the software Qcapture®. A millimetrically graduated ruler was attached to the captured images.
The software ImageTool 3.0® (University of Texas, San Antonio, USA) was used for measurement of the epithelial cell layer, according to the manufacturer's recommendations. The epithelial thickness was measured from the spinous layer to the boundary between the intermediate layer and superficial, and three measurements were performed in each of the three photographed fields. At every 10 measurements of epithelial thickness, one was repeated to assess intra-examiner reproducibility. All analyses were blind to the origin of the sample.
The presence of inflammatory infiltration was evaluated, and each microscopic field was labeled 0 or 1 according to absence or presence of inflammatory cells, respectively. For assessment of the number of blood vessels in each field, structures with endothelial lining and with red blood cells in their interior were counted. At every 10 fields, a count was repeated to assess intra-examiner reproducibility.
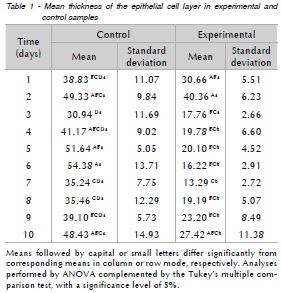
In order to compare epithelial thickness and number of blood vessels between control and experimental samples in the different periods, statistical analysis was accomplished by using multiple analysis of variance, complemented by the Tukey's multiple comparison test. P < 0.05 was taken to indicate statistical significance.
This work followed the rules for the use of animals in research projects, as Normative Resolution 04/97, the Committee for Research Ethics and Health/GPPG/HCPA. This project was approved by the Research Ethics Committee of the Dentistry Faculty, UFRGS.
Results
At macroscopic observation, the samples showed no alteration in the ventral tongue mucosa, with an index 0 in samples of experimental and control groups, in different periods.
Determination of the intraclass correlation coefficient showed an excellent intra examiner reproducibility in measurements of the epithelial layer thickness (ri = 0.999, p < 0.001) and counting of blood vessels (ri = 1.000).
As presented in Table 1, histological analysis shows that the highest mean epithelial thickness was observed in control samples collected on the sixth day and was significantly higher than samples collected on days one, three, seven, eight and nine. Fifth-day control samples presented mean epithelial thickness average significantly higher than samples collected on days three, seven and eight. Finally, control samples from days two and 10 also show significantly higher epithelial thickness means than samples on the third day. In the experimental group, mean epithelial thickness was highest on day-two samples. This result was significantly higher when compared to samples collected between days three and nine. Day-7 samples showed epithelial thickness significantly lower than sample collected on days one and two. Finally, samples collected from the fourth day on showed significantly lower epithelial thickness when compared to samples from the first three days. In the comparison between groups (Fig. 1), from the third day the average epithelial thickness of control samples is significantly higher than in experimental samples.
The mean number of blood vessels per field was similar in control and experimental samples collected in different periods. Inflammatory infiltrates were not observed in any of the control and experimental samples.
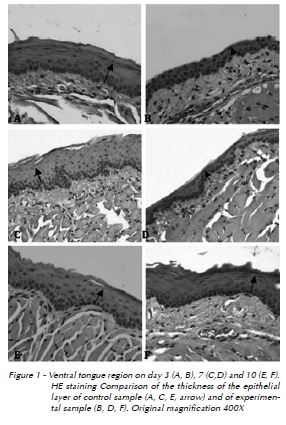
Discussion
Methotrexate, which is a drug widely used in bone marrow transplantation10,11, was used this study to induce mucositis in mice. The use of this drug for experimental work requires careful handling in a fume hood with special protective equipment, and involves a high cost. According to the methodology described by Vanderhoof et al.9 (1990) and Mota6 (2004), the total dose of the drug was administered during three consecutive days. In spite of resulting in a greater involvement by the researcher, this schedule reduces the toxicity of the drug, resulting in lower mortality of the experimental animals.
Since the ventral mucosal tongue region does not present a keratinized layer, it is more easily affected by chemotherapy16, and was thus chosen in this experimental study for assessing the consequences of methotrexate-induced mucositis.
In humans, mucositis manifests clinically between 5 and 7 days after commencing chemotherapy, presenting as ulcerative diffuse lesions5-7. In the present study, intraoral clinical manifestations were not observed in treated mice.
The phases of development of mucositis are interdependent2. In the present study, no significant differences related to the number of vessels were observed between control and experimental groups, and inflammatory cells were not seen in any of the samples in different periods. These results may be related to the methodology used.
In the epithelial phase of mucositis, the number of epithelial cells is reduced, resulting in atrophy and possibly leading to ulceration of the epithelium. These stages occur from day zero to the fifth day after administration of chemotherapy drugs in experimental animal studies2,6,22. The thickness of the epithelial cell layers observed in experimental samples collected on days three, four, five, six, seven and eight was significantly lower than those of control samples. The ulcerative or bacteriological phase of mucositis1,6 was not observed.
Oral mucositis is a multifactorial condition, so that the degree of myelosuppression, as well as environmental factors represented by the oral microbial flora, saliva and its protective/repairing components, added to the possible role of functional trauma, provide an important set of factors that impact on the frequency, severity and course of the disease2.
The repair phase, which is associated with renewal of the epithelium and increased thickness of the epithelial cell layer, could be observed from the eighth day onwards in experimental samples. This phase may last from 12 to 16 days, depending on the degree of epithelial proliferation, on the restoration of the oral microbiota and on the absence of complicating factors that may affect healing1,2.
It should be emphasized that, in a similar investigation, Sonis2 (1998) used 5-fluorouracil to induce mucositis in a hamster experimental model. These differences prevent a direct comparison with the present study, since repair processes of these two animal models are different, and the induction of mucositis depends on the drug and dose used.
The present study provides new information on the progression of methotrexate-induced mucositis and encourages the search for an efficient modulation of all phases of this condition.
Conclusion
This study showed that, in the mouse model, methotrexate-induced mucositis does not result in clinical manifestations in the ventral tongue region. No inflammatory infiltration was observed, and the average number of blood vessels was similar in control and experimental groups. However, the thickness of the epithelial cell layer was significantly lower in experimental as compared to control samples (p < 0.001), which may indicate alterations, although no clinical signs of ulceration and no histological evidence of rupture of the epithelial barrier and exposure of connective tissue were observed.
References
1. Peterson LJ, Ellis E, Hupp JR, Tucker MR. Cirurgia Oral e Maxilofacial Contemporânea. 4. ed. Rio de Janeiro: Elsevier; 2005. [ Links ]
2. Sonis ST. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol 1998; 34:39-43.
3. Clarke J, Butler R, Howarth G, Read L, Regester G. Exposure of oral mucosa to bioactive milk factors reduces severity of chemotherapy-induced mucositis in the hamster. Oral Oncol 2002; 38:478-85.
4. Leitão RF, Ribeiro RA, Bellaguarda EA, Macedo FD, Silva LR, Oriá RB, et al. Role of nitric oxide on pathogenesis of 5-fluorouracil induced experimental oral mucositis in hamster. Cancer Chemother Pharmacol 2007; 59:603-12.
5. Mitsuhashi H, Suemaru K, Li B, Cui R, Araki H. Evaluation of topical external medicine for 5-fluorouracil-induced oral mucositis in hamsters. Eur J Pharmacol 2006; 551(1- 3):152-5.
6. Mota MLS. Avaliação dos efeitos do agente citoprotetor amifostina na mucosite oral e disfunção da barreira intestinal. Modelos experimental em ratos e em pacientes portadores de câncer submetidos à quimioterapia antineoplásica [Tese de Doutorado]. Ceará: Faculdade de Medicina da UFC; 2004.
7. Morvan FO, Baroukh B, Ledoux D, Caruelle JP, Barritault D, Godeau G, et al. An engineered biopolymer prevents mucositis induced by 5-fluorouracil in hamsters. Am J Pathol 2004; 164(2):739-46.
8. Sonis ST, Van Vugt AG, McDonald J, Dotoli E, Schwertschlag U, Szklut P, et al. Mitigating effects of interleukin 11 on consecutive courses of 5-fluorouracil-induced ulcerative mucositis in hamsters. Cytokine 1997; 9:605-12.
9. Vanderhoof JA, Park JH, Mohammadpour H, Blackwood D. Effects. of dietary lipids on recovery from mucosal injury. Gastroenterology 1990; 98(5 Pt 1):1226-31.
10. Boente PC, Sampaio Filho CA, Giglio A. Agentes antineoplásicos. In: Penildon S, editor. Farmacologia. Rio de Janeiro: Guanabara Koogan; 2002. p. 1112-7.
11. Doroshow JH. Princípios da Oncologia Médica. In: Pollock RE, Doroshow JH, Khayat D, Nakao A, O´Sullivan B, editors. Manual de Oncologia Clínica da UICC – União Internacional Contra o Câncer. São Paulo: Fundação Oncocentro de São Paulo; 2006. p. 244-59.
12. Epstein JB, Schubert MM. Oral mucositis in myelosuppressive cancer therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999; 88(3):273-6.
13. Ferreira MCD, Santos PSS, Sabbagh-Haddad A. Condições sistêmicas: pacientes oncológicos submetidos à radioterapia e/ou quimioterapia. In: Sabbagh-Haddad A, editor. Odontologia para pacientes com necessidades especiais. 1. ed. São Paulo: Livraria e Editora Santos; 2007. p. 391-9.
14. Knox JJ, Puodziunas AL, Feld R. Chemotherapy-induced oral mucositis. Prevention and management. Drugs Aging 2000; 17(4):257-67.
15. Scully C, Sonis S, Diz PD. Oral mucositis. Oral Dis 2006; 12(3):229-41.
16. Varellis MLZ. Pacientes Oncológicos. In: O Paciente com necessidades especiais na Odontologia - Manual prático. São Paulo: Editora Santos; 2005. p. 459-70.
17. Stokman MA, Spijkervet FK, Wymenga AN, Burlage FR, Timens W, Roodenburg JL, et al. Quantification of oral mucositis due to radiotherapy by determining viability and maturation of epithelial cells. J Oral Pathol Med 2002; 31(3):153-7.
18. World Health Organization: WHO Handbook for reporting results of cancer treatment. 8. ed. Genebra: Offset Prints; 1979.
19. Mezadri TJ, Tomáz VA, Amaral VLL. Eutanásia Animal. In: Animais de Laboratório: Cuidados na iniciação experimental. 1. ed. Florianópolis: Editora da UFSC; 2004. p. 133-9.
20. Rhoden CR, Maslinkiewisz A, Pereira MSM, Rhoden EL. Eutanásia em animais de laboratório. In: Rhoden EL, Rhoden CR, editors. Princípios e técnicas em experimentação animal. 1. ed. Porto Alegre: Editora da UFRGS; 2006. p. 55-8.
21. Souza NL. Eutanásia. In: Luca RR, Alexandre SR, Marques T, Souza NL, Merusse JLB, Neves SP, editors. Manual para técnicos em bioterismo. 2. ed. São Paulo: FINEP-COBEA; 1996. p. 157-77.
22. Berger AM, Clark-Snow C. Adverse effects of treatment. In: Devita Jr VT, Hellman S, Rosenberg SA, editors. Cancer – Principles and practice of oncology. 6. ed. Philadelphia: Lippincott Williams & Wilkins; 2001.
 Endereço para correspondência:
Endereço para correspondência:
Edela Puricelli
Rua Quintino Bocaiúva, 465
90440-051 Porto Alegre - RS
e-mail: epuricelli@uol.com.br
Recebido: 30.12.2010
Aceito: 11.07.2011













