Serviços Personalizados
Artigo
Links relacionados
Compartilhar
RFO UPF
versão impressa ISSN 1413-4012
RFO UPF vol.17 no.1 Passo Fundo Jan./Abr. 2012
Osteoinduction of human bone marrow cells: an in vitro study
Introdução osteogênica de células da medula óssea humana: um estudo in vitro
Raphael Carlos Drumond LoroI; Denise Cantarelli MachadoII; Marília Gerhardt de OliveiraIII; Patrícia Wehmeyer FregapaniIV; Christian ViezzerV; Gilson Correia BeltrãoVI
IDDS, MSc, PhD, Associate Professor, Oral and Maxillofacial Surgery Department, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, RS, Brazil.
IIMSc, PhD, Associate Professor, School of Medicine, Biomedical Research Institute, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, RS, Brazil.
IIIDDS, MSc, PhD, Associate Professor, Oral and Maxillofacial Surgery Department, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, RS, Brazil.
IVDDS, PhD Student, Oral and Maxillofacial Surgery Program, Universidade Luterana do Brasil, Canoas, RS, Brazil.
VPhD student, Mining, Steel, and Materials Engineering, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
VIDDS, MSc, PhD, Surgeon, Oral and Maxillofacial Department; Hospital Municipal de Pronto Socorro, Porto Alegre, RS, Brazil.
ABSTRACT
Objective: This study evaluated the osteogenic induction of human bone marrow cells by human recombinant bone morphogenetic protein-4 (rhBMP-4) and proteins released by Saos-2 (human osteosarcoma cell line). Study design: Osteoinduction in the presence or absence of Saos-2 and/or rhBMP-4 was evaluated in cultured human bone marrow cells. Morphological aspects and bone protein markers (osteonectin, osteopontin, and osteocalcin) were analyzed on days 1, 2, 5, 8, 11 and 14. Osteonectin expression was evaluated using immunohistochemistry with anti-secreted protein acidic and rich in cystein (anti-SPARC) antibody. mRNA transcripts for osteopontin were determined using RT- -PCR with specific primers. Results: Bone marrow cells were adherent since the first day of culture and were positive for osteonectin. mRNA transcripts were detected in all culture conditions since the first day of culture. As human osteosarcoma cells are a source of additional growth they did not affect osteoinduction. rhBMP-4 up regulates osteoinduction during the first days of culture only. Osteoblasts were obtained from human bone marrow cells even in the absence of growth factors and showed a typical morphology. Cells derived from bone marrow can undergo osteoinduction in vitro in the absence of osteoinductive factors such as bone morphogenetic proteins. Conclusions: This study showed that an osteoblastic cell lineage may be obtained from human bone marrow derived from adherent cells, and that the presence of the rhBMP-4 seems to have an effect during the first stages of differentiation only.
Keywords: Stem cells. Immunohistochemistry. Osteoblasts. Stem cells.
RESUMO
Objetivo: este estudo avaliou a indução osteogênica de células da medula óssea humana por proteína-4 morfogenética óssea recombinante humana (rhBMP-4) e proteínas liberadas pela Saos-2 (linha de células do osteossarcoma humano). Metodologia: a osteoindução, na presença ou na ausência de Saos-2 e/ou rhBMP-4, foi avaliada em células cultivadas de medula óssea humana. Aspectos morfológicos e marcadores de proteínas ósseas (osteonectina, osteopontina e osteocalcina) foram analisados nos dias 1, 2, 5, 8, 11 e 14. A expressão da osteonectina foi avaliada usando imuno-histoquímica com proteína ácida antissecretada e rica em anticorpo cisteína (anti-SPARC). Transcrições de mRNA para osteopontina foram determinadas através de RTPCR, com primers específicos. Resultados: as células de medula óssea aderiram desde o primeiro dia da cultura e se mostraram positivas para osteonectina. Transcrições de mRNA foram detectadas em todas as condições de cultura, desde o primeiro dia. O fato de que células do osteossarcoma humano são fonte adicional do fator de crescimento não afetou a osteoindução. RhBMP-4 regulou a osteoindução apenas durante os primeiros dias da cultura. Osteoblastos foram obtidos a partir de células de medula óssea humana, mesmo na ausência de fatores de crescimento e apresentaram morfologia característica. Células derivadas de medula óssea podem sofrer osteoindução in vitro na ausência de fatores de osteocondução, tais como proteínas morfogenéticas ósseas. Conclusões: este estudo revelou que uma linhagem celular osteoblástica pode ser obtida a partir de células aderentes derivadas de medula óssea humana e que a presença de rhBMP-4 parece ter efeito apenas durante os primeiros estágios da diferenciação.
Palavras-chave: Células-tronco. Imunoistoquímica. Osteoblastos.
Introduction
Undifferentiated mesenchymal stem cells are pluripotent cells that can differentiate into various phenotypes and are a source of osteogenic Cells1,2. The osteogenic process involves maturation and proliferation of precursor primitive cells into functional osteoblasts. The bone cell lineages originated from undifferentiated mesenchymal cells give rise to osteoprogenitor cells, preosteoblasts, osteoblasts and osteocytes. The development of osteoblastic cells from stem cells occurs throughout a series of transitional events that can be characterized by several morphological, biochemical and molecular criteria.
Alternative strategies, created by tissue engineering, will allow the development of new tools for bone regeneration3. In recent years, the osteogenic potential of demineralized bovine bone matrix implants has been investigated in studies based on the hypothesis that mesenchymal cells can differentiate into osteoblasts and chondroblasts for new bone4. Isolation and expansion of stem or osteoprogenitor cells and appropriated osteoinduction factors that mimic a proper environment are essential components for bone reconstitution. Osteoblasts, chondrocytes, myocytes and adipocytes are derived fromundifferentiated mesenchymal cells or mesenchymal stem cells. During the differentiation process, progenitor cells acquire specific phenotypes under the control of regulatory factors5.
Molecules, known as bone morphogenetic proteins (BMPs), induce bone formation, as well as some growth and differentiation factors. BMPs have a major role in the differentiation of mesenchymal cells into osteoblasts6. These are osteoinductive cytokines, although some are not secreted molecules7. Bone formation is dependent on a number of microenvironmental signals, such as cytokines and growth factors, molecules from the extracellular matrix and cell-to-cell interactions that act in concert.
Ectopic bone formations have being described as a result of BMP inductions. Osteoblasts are believed to be the main source of BMP secretion and deposition into the extracellular matrix. Moreover, several studies are under way to examine the molecular mechanism of ectopic bone formation and the biological effects of recombinant BMPs on osteoblast differentiation through primary or lineage cell cultures6,8.
Human osteosarcoma cell lineages can synthesize, store and secrete several BMPs and induce bone formation9. Several studies are under way to identify the mechanisms of osteoinduction by those molecules7,10,11. The Saos-2 can mainly synthesize BMP-1, BMP-2, BMP-3, BMP-4, BMP-6 and TGF-β.
Osteopontin, osteonectin and osteocalcin are bone markers that can help to determine the several stages of osteogenic differentiation during the osteoinductive process and have been used for the differential diagnosis of osteosarcoma12. Osteonectin, also known as SPARC, is synthesized by osteoblasts in vitro, in the early phases of osteoblastic differentiation13. Moreover, this protein is found in several tissues undergoing remodeling and repair13,14. Osteopontin expression occurs in the early stages of differentiation during bone precursor proliferation and is produced in large amounts in osteoblasts15,16.
Once the mechanisms that involve differentiation of precursor cells into several cell types are known, surgical procedures to treat a variety of pathologies related to tissue neoformation and remodeling would be favored by a shortened period of healing, which may result in more successful surgical interventions. Moreover, several reports have described the isolation and expansion of bone-marrow-derived mesenchymal stem cells (MSCs). Some of them focused on scaffolds and their putative application to bone repair17, tooth bioengeneering18 and more effective osseointegration19.
Bone-marrow-derived cells seem to have the largest capability to differentiate into diverse cell types, including endothelium and myoblasts, and to became part of the neural system, the liver and the heart20.
This study evaluated the osteogenic induction of human bone marrow cells by human recombinant bone morphogenetic protein-4 (rhBMP-4) and proteins released by Saos-2 (human osteosarcoma cell line).
Material and methods
Material
Dulbeco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS) for cell culture, Histopaque®-1077 and DPBS were purchased from GIBCO® (Grand Island, NY, USA). Human recombinant bone morphogenetic protein-4 (rhBMP-4) was purchased from R&D Systems Inc. (Minneapolis, MN, USA). All other routine reagents were analytical grade.
Human bone marrow harvesting and preparation of mesenchymal cells
Human bone marrow cells were obtained after written informed consent from a patient undergoing maxillary reconstruction surgery. This study was approved by the Ethics Committee of Hospital São Lucas, Pontifícia Universidade Católica do Rio Grande do Sul, Brazil, protocol no 06/03481. Human bone marrow cells were aspirated with a syringe from the iliac crest of a 28-year-old woman. The cells were separated by centrifugation over a Histopaque®-1077 (GIBCO®, Grand Island, NY, USA) gradient at 400 g for 30 minutes. The mononuclear cell layer was aspirated, washed with 10 ml of DPBS (GIBCO®, Grand Island, NY, USA) by centrifugation at 500 g during 5 minutes and resuspended at 0.5 x 105 cell/ml with DMEM.
Saos-2 culture
Human osteosarcoma cell line (Saos-2) was purchased from the American Type Cell Culture Collection (HTB-85) (Manassas, VA, USA) and grown in DMEM culture medium supplemented with 10%
FBS.
Co-culture of human bone marrow derived cells with Saos-2
Osteoinduction of human bone marrow derived cells (HBMC) was performed using various conditions, as described in Table 1. Briefly, 2.5 ml of Saos-2 at a density of 0.5 x 105 cells/ml of DMEM was plated into 6 well plates to evaluate morphological and proliferative features. Immunohistochemistry was performed in cell cultures in 96 well plates seeded with 100 μl of cells at 0.5 x 105 cells/ml. Because Saos-2 is an adherent cell line, Saos-2 wasplated 24 hours before addition of mesenchymal cells when cells were co-cultured. Cell morphology and proliferation were observed at 1, 2, 5, 8, 11 and 14 days of culture.
Osteonectin detection by Immunohistochemistry
Anti-SPARC antibody (R&D Systems Inc., Minneapolis, MN, USA) at a concentration of 15 μg/ml was used to detect intracellular expression of osteonectin by cultured cells at 1, 2, 5, 8, 11 and
14 culture days. Briefly, the cells were washed with DPBS and fixed with 3.7% formaldehyde in DPBS for 10 min, incubated for 10 min with H2O2 to inhibit endogenous peroxidase, followed by methanol incuba tion for 6 min at –20 oC. After two washes with DPBS, cells were incubated with anti-goat immunoglobulin-biotin conjugated (Sigma, St. Louis, MO, USA) antibody (15 μg/ml) for 40 min at 37 oC. Cells were incubated with one drop of streptavidinperoxidase conjugate (Dako, Carpinteria, CA, USA) for 10 min at room temperature. After two washes with DPBS, the cells were incubated with 50 μl/well of a solution containing diaminobenzidine (Dako, Carpinteria, CA, USA). Finally, after a wash with DPBS, cells were analyzed under an inverted light microscope (Axiovert 25, Carl Zeiss AG, Oberkochen, Germany).
Reverse transcription polymerase chain reaction (RT-PCR) to detect osteopontin expression
Expression of osteopontin mRNA by HBMC, co-cultured or not with Saos-2 in the presence or absence of rhBMP-4 on days 1, 2, 5, 8, 11 and 14 were evaluated by RT-PCR. Total RNA was isolated from cultured cells using Trizol LS reagent (Invitrogen Inc., Carlsbad, CA, USA). First strand cDNA syntheses were carried out using oligo-dT12-18 primers (Invitrogen Inc., Carlsbad, CA, USA). After DNAse treatment of the samples, reverse transcription was carried out using Superscript II reverse transcriptase (Invitrogen Inc, Carlsbad, CA, USA). PCR reactions (50 μl) were performed with 5 μl of cDNA, using 2 U of Easy Taq-DNA polymerase (LabTrade do Brasil Ltda, São Paulo, SP, Brazil) and 25 pmol of osteopontin specific primers (forward: 5'CAT CTC AGA AGC AGA ATC TCC 3'; reverse: 5'CCA TAA ACC ACA CTA TCA CCT C 3') and run on a thermocycler (Peltier Thermal Cycler-200, MJ Research Inc., Waltham, MA, USA). The 35 cycles of PCR were performed as follows: denaturation step at 94 oC for 30 sec, annealing at 55 oC for 45 sec and extension at 72 oC for 1 min.
Results
Morphological and proliferative features of human bone marrow derived cells
Human bone marrow derived cells adhered after 24 hours in culture. However, they show proliferation and colony formation in the presence of rhBMP-4. These characteristics were not detected when they were co-cultured with Saos-2. Cell colonies in intense proliferation were observed in all conditions between the 5th and 8th culture days, and HBMC alone showed extensive communicating branches. Moreover, HBMC had multiple layers of interconnected cells after 14 culture days.
Osteonectin detection by Immunohistochemistry
Human bone marrow cells stained for osteonectin after 24 hours in culture as detected by immunohistochemistry. Co-cultures of HBMC and Saos-2 were both positive for anti-SPARC antibody, but HBMC always had a more intense staining. Cell differentiation and proliferation were confirmed with anti-SPARC antibody after five days. Cells with a distinct morphology were seen, as well as cells that were apparently growing in culture but that did not stain for osteonectin (Figure 1 A-D).
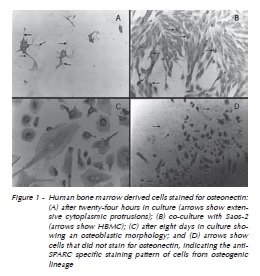
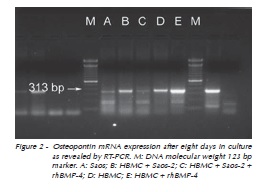
Osteopontin mRNA expression
Messenger RNA expression was revealed by RT-PCR using total RNA from culture cells as described. As expected, Saos-2 expressed mRNA for osteopontin from day 1. After 8 days(Figure 2), cultured human bone marrow derived cells expressed osteopontin mRNA. However, cultures supplemented with rhBMP-4 seemed to produce higher levels of osteopontin mRNA. Similarly, high expression levels of osteopontin were synthesized by cultured cells (HBMC and Saos-2) in the presence or not of rhBMP-4 on days 11 and 14 (Figure 3).

Discussion
Bone marrow derived cells can growth adherent or in suspension21,22. Cells that adhere can differentiate into mesenchymal cells, while cells that grow in suspension will give rise to hematopoietic cells. Several studies have confirmed the adhesion property of mesenchymal cells23-25 and we found that HBMCs are already attached to the culture substrate after 24 hours in culture.
Petite et al.25 (2000) have demonstrated that, after a few days, adherent bone marrow cells or mesenchymal cells in culture have three distinct morphological cell types, that is, compact spindleshaped, slender cells with long cytoplasmic extensions, and large and amorphous cells with many cytoplasmic extensions, as described in this study.
Bone morphogenetic proteins are molecules that can induce osteogenesis, and rhBMP-4 has the property to induce cell aggregates and colony formation, mainly in the first five days in culture26-28. The effect of rhBMP-4 on HBMC co-cultured with Saos-2 seems to be intensified, probably due to the fact that the osteosarcoma cell line Saos-2 also expresses and secretes BMP-4 and other BMPs9. In contrast, non-adherent cells decreased in number after five days in culture, probably because they are hematopoietic precursor cells that require other growth factors to undergo differentiation in culture, as already described in the literature23,25. Cocultures of HBMC with Saos-2 were not affected by rhBMP-4 after a longer period of culture, which is in agreement with observations made by Virdi et al.28 (1998), who showed an inhibition of mature osteoblast proliferation in the later phases of osteogenesis. Therefore, the differentiated cells obtained in this study may be classified as osteoblasts. In fact, mesenchymal adherent cells reached a stage at which they did not undergo further proliferation. Similar results10,15,16 have shown that cultured osteoprogenitor cells have a limited self-renewing capability.
Osteonectin, also known as SPARC, is characteristic of bone lineage cells, whose expression occurs from the preosteoblastic to the osteoblastic phase. The osteosarcoma and adherent mesenchymal cells studied here expressed osteonectin, as demonstrated by immunohistochemistry, which additionally confirms their osteoblastic origin, although adherent HBMC seems to produce higher levels of osteonectin. Our findings are confirmed by studies that used immunohistochemistry or RT-PCR techniques29.
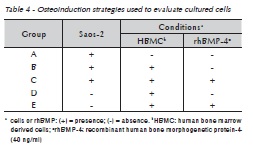
Moreover, adherent mesenchymal cells produced mRNAs for osteopontin in all conditions tested. Some studies have described a detectable expression of osteopontin in the early stages of osteogenesis with higher levels from the preosteoblastic to the osteoblastic stage15,16. This finding suggested that, after eight days in culture, adherent mesenchymal cells reached the preosteoblastic or osteoblastic phase of osteogenesis.
In the near future, autologous bone marrow cells will be applied to bone reconstitution, and the knowledge of their physiology and adequate manipulation, together with appropriate scaffolding techniques, will be necessary to restore bone structures in vitro and to replace damaged bone tissues, which will reduce the morbidity of this type of treatment.
Conclusions
This preliminary study results suggests that an osteoblastic cell lineage can be obtained from human bone marrow derived adherent cells even in the absence of osteoinductive factors such as BMPs, and that rhBMP-4 seems to have an effect only during the early differentiation stages. Human bone marrow stromal cells have an osteogenic potential and are prone to undergoing osteogenesis in short term cultures.
Acknowledgements
The authors thank Dr. Raphael Ott for providing the human bone marrow cells and Vinicius Schenk Michaelsen for the help with cell cultures. This study was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES - Brazil) and 3i Implants, Brazil.
References
1. Conrad C, Huss R. Adult stem cell lines in regenerative medicine and reconstructive surgery. J Surg Res. 2005; 124:201-8. [ Links ]
2. Schliephake H, Knebel JW, Aufderheide M, Tauscher M. Use of cultivated osteoprogenitor cells to increase bone formation in segmental mandibular defects: an experimental pilot study in sheep. Int J Oral Maxillofac Surg. 2001; 30:531-7. [ Links ]
3. Sonoyama W, Coppe C, Gronthos S, Shi S. Skeletal stem cells in regenerative medicine. Curr Top Dev Biol. 2005; 67:305-23. [ Links ]
4. Leite FR, Ramalho LT. Bone regeneration after demineralized bone matrix and castor oil (Ricinus communis) polyurethane implantation. J Appl Oral Sci. 2008; 16:122-6. [ Links ]
5. Partridge K, Yang X, Clarke NM, Okubo Y, Bessho K, Sebald W, et al. Adenoviral BMP-2 gene transfer in mesenchymal stem cells: in vitro and in vivo bone formation on biodegradable polymer scaffolds. Biochem Biophys Res Commun. 2002; 292:144-52. [ Links ]
6. Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002; 8:147-59. [ Links ]
7. Yoon ST, Boden SD. Osteoinductive molecules in orthopaedics: basic science and preclinical studies. Clin Orthop Relat Res. 2002; (395):33-43. [ Links ]
8. Gamradt SC, Lieberman JR. Genetic modification of stem cells to enhance bone repair. Ann Biomed Eng. 2004; 32:136-47. [ Links ]
9. Raval P, Hsu HH, Anderson HC. Osteoinductive ability of confluent Saos-2 cell correlates with enhanced expression of bone morphogenetic proteins. J Orthop Res. 1996; 14:605-10. [ Links ]
10. Muschler GF, Midura RJ. Connective tissue progenitors: practical concepts for clinical applications. Clin Orthop Relat Res. 2002; (395):66-80. [ Links ]
11. Strayhorn CL, Garrett JS, Dunn RL, Benedict JJ, Somerman MJ. Growth factors regulate expression of osteoblastassociated genes. J Periodontol. 1999; 70:1345-54. [ Links ]
12. Fanburg-Smith JC, Bratthauer GL, Miettinen M. Osteocalcin and osteonectin immunoreactivity in extraskeletal osteosarcoma: a study of 28 cases. Hum Pathol. 1999; 30:32-8. [ Links ]
13. Motamed K. SPARC (osteonectin/BM-40). Int J Biochem Cell Biol. 1999; 31:1363-6. [ Links ]
14. Meyer U, Meyer T, Vosshans J, Joos U. Decreased expression of osteocalcin and osteonectin in relation to high strains and decreased mineralization in mandibular distraction osteogenesis. J Craniomaxillofac Surg. 1999; 27:222-7. [ Links ]
15. Aubin JE. Advances in the osteoblast lineage. Biochem Cell Biol. 1998; 76:899-910. [ Links ]
16. Aubin JE. Bone stem cells. J Cell Biochem Suppl. 1998; 30-31:73-82. [ Links ]
17. De Kok IJ, Drapeau SJ, Young R, Cooper LF. Evaluation of mesenchymal stem cells following implantation in alveolar sockets: a canine safety study. Int J Oral Maxillofac Implants. 2005; 20:511-8. [ Links ]
18. Young CS, Abukawa H, Asrican R, Ravens M, Troulis MJ, Kaban LB, et al. Tissue-engineered hybrid tooth and bone. Tissue Eng. 2005; 11:1599-610. [ Links ]
19. Yamada Y, Ueda M, Naiki T, Nagasaka T. Tissue-engineered injectable bone regeneration for osseointegrated dental implants. Clin Oral Implants Res. 2004; 15:589-97. [ Links ]
20. Yen AH, Sharpe PT. Stem cells and tooth tissue engineering. Cell Tissue Res. 2008; 331:359-72. [ Links ]
21. Bertram H, Mayer H, Schliephake H. Effect of donor characteristics, technique of harvesting and in vitro processing on culturing of human marrow stroma cells for tissue engineered growth of bone. Clin Oral Implants Res. 2005; 16:524-31. [ Links ]
22. Chen J, Sotome S, Wang J, Orii H, Uemura T, Shinomiya K. Correlation of in vivo bone formation capability and in vitro differentiation of human bone marrow stromal cells. J Med Dent Sci. 2005; 52:27-34. [ Links ]
23. Aubin JE. Osteoprogenitor cell frequency in rat bone marrow stromal populations: role for heterotypic cell-cell interactions in osteoblast differentiation. J Cell Biochem. 1999; 72:396-410. [ Links ]
24. Krebsbach PH, Kuznetsov SA, Bianco P, Robey PG. Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med. 1999; 10:165-81. [ Links ]
25. Petite H, Viateau V, Bensaid W, Meunier A, de Pollak C, Bourguignon M, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000; 18:959-63. [ Links ]
26. Kessler S, Kastler S, Mayr-Wohlfart U, Puhl W, Gunther KP. Stimulation of primary osteoblast cultures with rh-TGF-beta, rh-bFGF, rh-BMP 2 and rx-BMP 4 in an in vitro model. Orthopade. 2000; 29:107-11. [ Links ]
27. Li G, Berven S, Simpson H, Triffitt JT. Expression of BMP-4 mRNA during distraction osteogenesis in rabbits. Acta Orthop Scand. 1998; 69:420-5. [ Links ]
28. Virdi AS, Cook LJ, Oreffo RO, Triffitt JT. Modulation of bone morphogenetic protein-2 and bone morphogenetic protein-4 gene expression in osteoblastic cell lines. Cell Mol Biol (Noisy-le-grand). 1998; 44:1237-46. [ Links ]
29. Malaval L, Modrowski D, Gupta AK, Aubin JE. Cellular expression of bone-related proteins during in vitro osteogenesis in rat bone marrow stromal cell cultures. J Cell Physiol. 1994; 158:555-72. [ Links ]
 Address for Correspondence:
Address for Correspondence:
Marília Gerhardt de Oliveira
Av. Coronel Lucas de Oliveira, 1841/203,
Bairro Petrópolis
90460.001 Porto Alegre - RS
e-mail: gerhardtoliveira@gmail.com
Recebido: 24/10/2011
Aceito: 19/03/2012













