Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Arquivos em Odontologia
versão impressa ISSN 1516-0939
Arq. Odontol. vol.47 no.2 Belo Horizonte Abr./Jun. 2011
Alkali soluble fluoride deposits on bovine dental enamel after treatment with a mouthwash containing fluoride and chlorhexidine
Deposição de fluoreto solúvel em álcali no esmalte dental bovino após tratamento com um enxaguatório contendo fluoreto e clorexidina
Isabel Cristina Gavazzoni Bandeira de AndradeI,II; Ilione Kruschewsky Costa Sousa OliveiraI; Priscila Leite Fahel GuimarãesI; Bruna Krouwel PeresI; Fabiana Mantovani Gomes FrançaI; Roberta Tarkany BastingI; Ynara Bosco de Oliveira Lima-ArsatiIII
I Curso de Odontologia, Faculdade São Leopoldo Mandic, Campinas, SP, Brazil
II Fundação Universitária Regional de Blumenau, Blumenau, SC, Brazil
III Departamento de Ciências Biológicas, Universidade Estadual de Feira de Santana (UEFS), Feira de Santana, BA, Brazil
Contato: andrade.isabel@terra.com.br, ilionek@uol.com.br, priscila.fahel@gmail.com, bru_kp@uol.com.br, biagomes@yahoo.com, rbasting@yahoo.com, ynaralima@yahoo.com
ABSTRACT
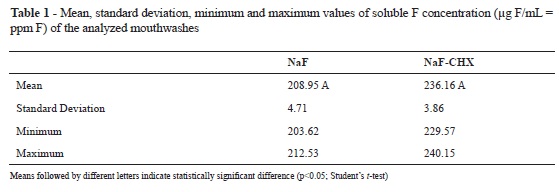
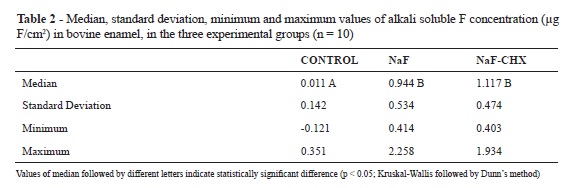
Aim: The aim of this in vitro study was to determine whether the addition of chlorhexidine (CHX) to a mouthwash containing fluoride (F) diminishes the concentration of soluble F and its reactivity with bovine dental enamel. Materials and Methods: First, the concentration of F found in mouthwashs was determined by an ion-specific electrode. To test their reactivity, 30 slabs of bovine dental enamel (5 x 5mm) were distributed into three groups (n = 10), according to the treatment applied, for 30 seconds: control group (distilled deionized water); NaF group (0.05% sodium fluoride); NaF + CHX group (Noplak Max®: 0.05% sodium fluoride + 0.12% chlorhexidine digluconate). After, the slabs were washed with distilled deionized water and individually immersed in artificial saliva for 30 minutes. They were dried and then individually immersed in 0.5 mL of 1M KOH for 24 hours, under agitation. After buffering the samples with HCI containing TISAB II, the concentration of F present in the KOH solutions was determined be means of an ion-specific electrode. Results: No significant difference in the concentration of soluble F in the studied mouthwashs (NaF and NaF+CHX; Student's t-test, p > 0.05) could be observed. Furthermore, no difference could be identified among the alkali soluble fluoride deposits from the NaF and NaF+CHX groups, and both groups presented higher deposit levels than did the control group (Kruskal-Wallis and Dunn´s method, p < 0.05). Conclusion: The addition of CHX to a mouthwash containing F did not decrease its soluble F concentration, nor its reactivity to bovine dental enamel.
Uniterms: Fluorides. Topical. Chlorhexidine. Mouthwash.
RESUMO
Objetivo: O objetivo deste estudo in vitro foi determinar se a adição de clorexidina (CHX) a um enxaguatório contendo fluoreto (F) diminui a concentração de F solúvel e a sua reatividade com o esmalte dental bovino. Materiais e Métodos: Inicialmente, a concentração de F nos enxaguatórios foi determinada pelo método do eletrodo específico. Para avaliar sua reatividade, 30 fragmentos de esmalte dental bovino (5x5mm) foram distribuídos em três grupos (n = 10), de acordo com o tratamento aplicado, por 30 segundos: grupo controle (água destilada e deionizada), grupo NaF (fluoreto de sódio 0,05%), grupo NaF+CHX (Noplak Max®: fluoreto de sódio 0,05% + digluconato de clorexidina 0,12%). Em seguida, os fragmentos foram lavados com água destilada e deionizada e imersos individualmente em saliva artificial por 30 minutos. Foram secados e então imersos individualmente em 0,5 ml de KOH 1M por 24h, sob agitação. A concentração de F nas soluções de KOH foi determinada pelo método do eletrodo específico, após tamponamento das amostras com TISAB II contendo HCl. Resultados: Não houve diferença significativa na concentração de F solúvel nos enxaguatórios avaliados (NaF e NaF+CHX; teste t de Student, p > 0,05). E não houve diferença na deposição de fluoreto solúvel em álcali nos grupos NaF e NaF+CHX; ambos os grupos obtiveram maior deposição do que o grupo controle (Kruskal-Wallis e Método de Dunn, p < 0,05). Conclusão: A adição de CHX a um enxaguatório contendo F não diminuiu sua concentração de F solúvel nem mesmo sua reatividade com o esmalte dental bovino.
Unitermos: Fluoretos tópicos. Clorexidina. Enxaguatórios.
INTRODUCTION
According to a Brazilian dental survey carried out in 2000, dental caries and periodontal disease have proven to be the most prevalent pathological manifestations in the oral cavity. These two oral diseases are the main factors responsible for tooth loss in Brazilian adults1. Among the different strategies proposed for caries control, the treatments based on the disorganization of the dental biofilm associated with the use of fluoride (F) have been considered effective2.
To understand the fluoride's mechanism of action, it is important to explain the chemical interactions that occur between the teeth (mainly enamel) and the oral fluids. Under physiological conditions, calcium and inorganic phosphate are found in oral fluids or biofilm in a supersaturated condition with respect to dental enamel, which mainly consists of hydroxiapatite crystals [Ca10(PO4)6OH2]. Under these conditions, saliva is able to remineralize the dental enamel. When the pH level drops, the solubility of hydroxiapatite increases considerably3. If ionic F is available in the oral environment, a reduction in the demineralization and an enhancement in the remineralization process occur, given that F induces mineral precipitation on the tooth structure in the form of fluorapatite [Ca10(PO4)6F2]. Therefore, it can be concluded that F slows down caries progression2.
Products that contain over 100 ppm F in their composition, such as mouthwashs, may induce the formation of calcium fluoride (CaF2) deposits (also called loosely-bound fluoride or alkali soluble fluoride) on the tooth surface. When a demineralizing condition exists, these deposits act as a reservoir for F, as they slowly release F and calcium, while the pH levels drop. Dentifrices, gels, varnishes, and mouthwashs are the most commonly used fluoridated products for this purpose3. The efficacy of fluoridated products to form calcium fluoride deposits depends on its F concentration (the greater the concentration, the greater the deposit), the type of F applied (stannous, sodium, among others), the time of exposure (the greater the time, the greater the deposit), and the pH of the product (the lower the pH, the greater the deposit)3,4.
Chlorhexidine (CHX) is a bactericidal chemical compound for topical use that was synthesized within a laboratory. In general, it is effective against gram-positive, gram-negative, facultative aerobic, and anaerobic bacteria, as well as other microorganisms, such as yeasts and fungi5. To date, CHX represents the most studied and efficient antimicrobial agent used to inhibit the formation and pathogenicity of bacterial dental biofilm. Prior studies have demonstrated that a single mouthwash with 0.2% chlorhexidine reduces the oral microbiota by 80-95%6, given that chlorhexidine can inhibit the metabolism of the biofilm as well as the activity of glucosyltransferase enzymes (responsible for the production of extra-cellular polysaccharides, which are important in microbial adhesion)7.
After a systematic review of the literature, it was found that the use of CHX (in the form of gel, dentifrice, or mouthwash) produced a cariesinhibiting effect of 46% in individuals of 11 to 15 years of age who are highly susceptible to caries disease8. It is important to note that the authors of the present study recognize the possibility of bias in their review due to the small number of studies involved in the meta-analysis, which may have led to an overestimation of the overall result.
In view of the effectiveness of F and CHX, an association of the two agents for the control of caries and gingivitis was proposed more than thirty years ago. The results of this association have been controversial. Prior studies have reported a synergic toxic effect on bacterial cytoplasm, as well as on enzymes responsible for carbohydrate fermentation, in turn diminishing the formation of acid by the microorganisms9-11, which was reflected in the effective control of dental caries12,13. Nevertheless, a decrease in the substantivity of CHX, from a commercialized mouthwash brand, could be identified in vitro when associated with sodium fluoride14.
It is also important to point out that, to be associated with CHX, F must appear in the form of a sodium fluoride. The use of sodium monofluorphosphate, a form of fluoride commonly used in dentifrices, is not recommended, since it interacts with CHX, forming low-solubility salts and consequently decreasing its action15.
However, it has been reported that the association between sodium fluoride and 0.12% chlorhexidine digluconate is compatible and is capable of inhibiting biofilm formation as well as of incorporating F within the dental enamel in the form of fluorapatite, after performing an in situ cariogenic challeng16. However, this result has not yet been proven, especially as concerns the formation of calcium fluoride deposits. As the effectiveness of high concentrations of F in caries control is directly dependent on the formation of these deposits on the surface of the enamel, it is relevant to evaluate whether or not this reaction is in fact affected by its association with chlorhexidine.
Therefore, the present in vitro study aimed to determine whether or not the addition of chlorhexidine to a fluoridated mouthwash was able to reduce the concentration of soluble F and the formation of calcium fluoride deposits on tooth enamel.
MATERIALS AND METHODS
The research was approved by the Ethics and Animal Experimentation Committees of the School of Dentistry at São Leopoldo Mandic.
The mouthwashs used in this study included:
• 0.05% NaF (sodium fluoride; 225 ppm F): manufactured by Flora, compounding pharmacy, Brazil (CNPJ:02028407/0001-52);
• Noplak Max (0.12% chlorhexidine digluconate + 0.05% sodium fluoride): manufactured by Daudt Laboratory Ltda., Brazil.
The concentration of F in the products was determined by using a specific F electrode (Orion 9609 ionplus Fluoride electrode, Thermo electron corporation, USA) coupled to a potentiometer (RbPH - 210, MS Tecnopon Equip. Especiais Ltda, Brazil), previously calibrated with standards containing 12.5 ppm F, 25 ppm F, 50 ppm F, and 100 ppm F. The mouthwash samples were diluted four times with distilled deionized water (one part mouthwash: three parts water). Both the standards and samples were buffered with 50% TISAB II (total ionic strength adjustment buffer; 1.0 M acetate buffer, pH 5.0, containing 1.0 M NaCl, 0.4% CDTA, and 20g of NaOH/L), which suited the pH and ionic forces of the samples. All analyses were performed in triplicate.
Alkali soluble fluoride deposit on bovine dental enamels
Preparation of bovine dental slabs
Thirty slabs of bovine dental enamel, measuring 5 x 5 mm, were taken from approximately fifteen bovine lower incisors, which were previously stored for 30 days in glass recipients containing a 0.1% aqueous thymol solution (Labsynth, Brazil). Thereafter, the slabs were covered with sticky wax (Babinete, Brazil), leaving only the vestibular surface uncovered.
Treatment of slabs with mouthwashs
The slabs were randomly divided into 3 groups, totaling 10 slabs in each group, according to the treatment applied (immersion of all the slabs of the same group in 200 mL of the respective solutions, 0.8 mL/mm2 enamel area, during 30 seconds):
• Control Group: distilled deionized water.
• Group NaF: 0.05% Sodium Fluoride (225 ppm F).
• Group NaF-CHX: 0.05% Sodium Fluoride + 0.12% Chlorhexidine Digluconate (Noplak MaxTM).
After treament, the slabs were rinsed with distilled deionized water for one minute and kept in 20 mL of artificial saliva (1.5mM Ca, 0.9mM P, 150mM KCl, 20 mM tri-hydroxymethylaminomethane buffer, pH = 7.0)17 for 30 minutes, as suggested by the manufacturer of the mouthwashs. The slabs were dried with absorbent paper before the CaF2 extraction was performed.
Extraction and dosage of alkali soluble fluoride from the slabs
CaF2 extraction was performed according to Caslavska et al.18, in which each fragment was placed in a microtube (Safe-Lock, Eppendorf, USA) containing 0.5 mL of 1M KOH. Each microtube was closed, the samples were agitated for 24 hours in a plate shaker (Rock CT-158, Cientec, Brazil), and two aliquots (0.2 mL each) were obtained for F analysis.
After buffering the samples with an identical volume of 1M HCl containing TISAB II, fluoride analyses were performed using the specific F electrode. These analyses were performed in duplicate.
The values of F concentrations in the solutions (μg F/mL) were divided by the area in cm2 (5 x 5 mm2 = 25 mm2 = 0.25 cm2), resulting in values of μg F/ cm2.
Statistical analysis
The results of F concentration in the two mouthwashs were analyzed by means of the Student's t-test. The results of alkali soluble F extracted from the enamel slabs in the three groups were analyzed by means of the non-parametric Kruskal-Wallis test, followed by the Dunn's method, due to the non-homogeneity of their variances. The level of significance was established at 5% (p < 0.05).
RESULTS
No significant difference in the concentration of soluble F could be observed among the studied mouthwashs (NaF and NaF+CHX; Student's t-test, p > 0.05; Table 1). Furthermore, no difference could be identified among the alkali soluble fluoride deposits from the NaF and NaF+CHX groups, and both groups presented higher deposit levels than did the control group (Kruskal-Wallis and Dunn´s method, p < 0.05 (Kruskal-Wallis followed by Dunn's method, p < 0.05; Table 2).
DISCUSSION
The use of mouthwashs is justified under special conditions, in which a high susceptibility to dental caries and periodontal disease can be identified, such as in cases in which it is difficult to control biofilm, or where the salivary flow rate is reduced19. For this reason, many different mouthwashs are available on the market today.
Products containing F act by controlling the caries process, slowing down demineralization, activating remineralization20, and producing antimicrobial activity21. Their role in reducing the prevalence of caries worldwide is also wellrecognized20. Chlorhexidine is an extremely efficient antimicrobial agent in the reduction of dental biofilms5. However, the association of F and CHX in the same product continues to be discussed throughout scientific literature, especially as concerns their effectiveness11,14-16,19.
In the present in vitro study, it could be observed that the concentration of soluble F in the two products was within the value reported in the label, leading us to the conclusion that the addition of CHX to the F mouthwash did not alter its soluble F concentration. As regards the alkali soluble F deposit on the bovine dental enamel slabs, the obtained results are in agreement with the literature16, showing that the association of CHX with sodium fluoride did not affect its reactivity with the dental enamel.
Nevertheless, data in the literature that can be directly compared to the present in vitro study is scarce, because there are many differences among the employed methodologies. Hayacibara et al.22 reported that the formation of CaF2 deposits on bovine enamel, after treatment with gel, foam, and varnish, were respectively 31.72, 44.57, and 22.06 μg F/cm2 (mean values). These values are significantly higher than those obtained in the present study. However, Hayacibara et al.22 studied products containing up to 100 times more F (12.642.5, 12.756.0, and 23.183.2 ppm F, respectively; mean values) than did the present study. In addition, these did not immerse the slabs in artificial saliva for 30 minutes after the treatments. Tabchoury et al.23 and Arthur et al.24 also evaluated the reactivity of F from mouthwashs to bovine enamel; however, these authors analyzed the formation of incorporated F in enamel (fluorapatite, "firmly bound"), and the treatments were applied for only 10 minutes.
The in situ study of Del Bel Cury et al.16 mentioned that there was an increase in the concentration of F incorporated within the enamel (fluorapatite, "firmly bound"), and the reduction in biofilm formation when the association of a mouthwash containing 0.12% CHX and 0.05% NaF was used. The in vitro study of Luoma et al.12 also found that the pre-treatment of dental enamel with CHX did not hinder the effect of F, also allowing a reduction in dental biofilm formation. These data agree with the results from a clinical study conducted by Duarte et al.25, who evaluated the effect of adding 0.12% chlorhexidine digluconate (CHX) to a 0.05% (NaF) mouthwash in arresting active enamel caries lesions. The authors concluded that the addition of CHX did not improve the arrestment capacity of the NaF mouthwash.
Concerning the doubt about whether F could affect the antimicrobial effect of CHX, Freitas et al.14 evaluated the in vitro substantivity of CHX and whether or not it was in fact associated with NaF at different time intervals. The results showed that the concentration of CHX was significantly reduced when it was associated with NaF, suggesting that this association presented no beneficial effect. A reduction in the concentration of CHX was also reported when it was associated with monofluorphosphate (MFP), thereby decreasing the efficacy of both products15.
Thus, in the present study, no interaction was found between 0.05% NaF and 0.12% chlorhexidine digluconate in relation to the solubility and reactivity of F. As such, it could be concluded that the association F-CHX is feasible in these aspects. However, to better defend this claim, additional studies, using standard methodologies and evaluating the antimicrobial effect of CHX, are warranted.
Furthermore, it is known that the prolonged use of a mouthwash containing 0.05% sodium fluoride presents no side effects and is very important in controlling dental caries26. Nevertheless, the prolonged use of 0.12% chlorhexidine has undesirable side effects, such as: staining the acquired pellicle, loss of taste, metallic taste, and alteration in the oral microbiota, in turn favoring the proliferation of fungi27. Therefore, certain precautions must be taken when recommending these products: recommend a safe period of time for this treatment process, recommended only for patients with a high number of caries and/or with a high risk of periodontal disease, and recommend this treatment only for children of more than 7 years of age. Finally, it is important to consider the affirmation of Cury & Tenuta2 that any strategy to reduce the progression of dental caries (including periodontal disease) should be based on its control as a biofilm-dependent disease.
CONCLUSIONS
The addition of 0.12% chlorhexidine to a fluoridated mouthwash did not reduce the concentration of soluble fluoride within it;
The addition of 0.12% chlorhexidine to a fluoridated mouthwash did not reduce the reactivity of fluoride with in vitro bovine dental enamel surfaces.
REFERENCES
1. Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Ce EIF, Fejerskov O. Chemical interactions between the tooth and oral fluids. In: Fejerskov O, Kidd E. Dental caries. The disease and its clinical management. 2nd ed. Oxford: Blackwell Munksgaard; 2008. chap. 12. [ Links ]
2. Cury JA, Tenuta LM. Enamel remineralization: controlling the caries disease or treating early caries lesions? Braz Oral Res. 2009; 23 Suppl 1:23-30.
3. Ten Cate JM, Larsen MJ, Pearce EIF, Fejerskov O. Chemical interactions between the tooth and oral fluids. In: Fejerskov O, Kidd E. Dental caries. The disease and its clinical management. 2nd ed. Oxford: Blackwell Munksgaard; 2008. chap. 12.
4. Saxegaard E, Rölla G. Fluoride acquisition on and in human enamel during topical application in vitro. Scand J Dent Res. 1988; 96:523-35.
5. Armitage GC. Biological bases for periodontal therapy. 2nd ed. São Paulo: Santos; 1993.
6. Schiott CR. Effect of chlorhexidine on the microflora of the oral cavity. J Periodontal Res. 1973; 12:7-10.
7. Scheie AA, Eggen KH, Rolla G. Glucosyltransferase activity in human in vivo formed enamel pellicle and in whole saliva. Scand J Dent Res. 1987; 95:212-5.
8. Van Rijkom HM, Truin GJ, Van‘t Hof MA. A meta-analysis of clinical studies on the cariesinhibiting effect of chlorhexidine treatment. J Dent Res. 1996; 75:790-5.
9. Emilson CG, Krasse B, Westergren G. Effect of a fluoride-containing chlorhexidine gel on bacteria in human plaque. Scand J Dent Res. 1976; 84: 56-62.
10. McDermid AS, Marsh PD, Keevil CW, Ellwood DC. Additive inhibitory effects of combinations of fluoride and chlorhexidine on acid production by Streptococcus mutans and Streptococcus sanguis. Caries Res. 1985; 19:64-71.
11. Meurman JH. Ultrastructure, growth and adherency of Streptococcus mutans after treatment with chlorhexidine and fluoride. Caries Res. 1988; 22:283-7.
12. Luoma HH, Murtomaa H, Nuuja T, Nyman A, Nummikoski P, Ainamo J et al. A simultaneous reduction of caries and gingivitis in a group of schoolchildren receiving chlorhexidine-fluoride applications:results after 2 years. Caries Res. 1978; 12:290-8.
13. Katz S. The use of fluoride and chlorhexidine for the prevention of radiation caries. J Am Dent Assoc. 1982; 104:164-70.
14. Freitas CS, Diniz HFO, Gomes JB, Sinisterra RD, Cortés ME. Evaluation of the substantivity of chlorhexidine in association with sodium fluoride in vitro. Pesqui Odontol Bras. 2003; 17: 78-81.
15. Barkvoll GR, Rölla G, Bellagamba S. Interaction between chlorhexidine digluconate and sodium monofluorophosphate in vitro. Scand J Dent Res. 1988; 96:30-3.
16. Del Bel Cury AA, Rebelo MAB, Cury JA. Efeito do bochecho com clorexidina (CH) e flúor (F) na redução de formação de placa dental e incorporação de flúor no esmalte dental. Rev Bras Odontol. 1994; 51: 26-9.
17. Serra MC, Cury JA. The in vitro effect of glass ionomer cement restoration on enamel subjected to demineralization and remineralization model. Quintessence Int. 1992; 23:143-7.
18. Caslavska V, Moreno EC, Brudevold F. Determination of the calcium fluoride formed from in vitro exposure of human enamel to fluoride solutions. Arch Oral Biol. 1975; 20:333- 9.
19. Featherstone JD. Delivery challenges for fluoride, chlorhexidine and xylitol. BMC Oral Health. 2006; 6:S8.
20. Tenuta LMA, Cury JA. Fluoride use in Dentistry and its anti-caries mechanism: part I. J Assoc Bras Odontol. 2008. [updated 2010 Jun 29; cited 2010 Sept 13]. Available at: http://www.abo.org.br/jornal/115/artigo1.php
21. Koo H. Strategies to enhance the biological effects of fluoride on dental biofilms. Adv Dent Res. 2008; 20:17-21.
22. Hayacibara MF, Leme AFP, Lima YBO, Gonçalves NCAV, Queiroz CS, Gomes MJ, Kozlowski F. Alkali-soluble fluoride deposition on enamel after professional application of topical fluoride in vitro. J Appl Oral Sci. 2004; 12:18-21.
23. Tabchoury CPM, Pierobon CN, Cury JA. Concentration and bioavailability of fluoride in mouthrinses prepared in dispensing pharmacies. J Appl Oral Sci. 2005; 13:41-6.
24. Arthur RA, Tabchoury CPM, Giancristófaro M, Del Bel Cury AA, Cury JA. Effect of preservatives on reactivity of fluoride with dental enamel. Rev Gaucha Odontol. 2007; 55:375-9.
25. Duarte AR, Peres MA, Vieira RS, Ramos-Jorge ML, Modesto A. Effectiveness of two mouth rinses solutions in arresting caries lesions: a short-term clinical trial. Oral Health Prev Dent. 2008; 6:231-8.
26. Poulsen S. Fluoride-containing gels, mouth rinses and varnishes: an update of evidence of efficacy. Eur Arch Paediatr Dent. 2009; 10:157-61.
27. Jenkins S, Addy M, Newcombe R. Evaluation of a mouthrinse containing chlorhexidine and fluoride as an adjunct to oral hygiene. J Clin Periodontol. 1993; 20:20-5.
 Author for correspondence:
Author for correspondence:
Ynara Bosco de Oliveira Lima-Arsati
Universidade Estadual de Feira de Santana, Departamento de Ciências Biológicas
Avenida Transnordestina, s.n., Bairro Novo Horizonte
CEP: 44.036-900 - Feira de Santana - BA - Brazil
E-mail: ynaralima@yahoo.com
Received on 25/01/2011 - Accepted on 06/05/2011













