Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Arquivos em Odontologia
versão impressa ISSN 1516-0939
Arq. Odontol. vol.49 no.2 Belo Horizonte Abr./Jun. 2013
Microbial susceptibility of bacteria and fungi against extracts of Psidium guajava L.: in vitro evaluation
Susceptibilidade microbiana de bactérias e fungos frente ao extrato de Psidium guajava L.: avaliação in vitro
Rafael Menezes Silva I; Maurício da Rocha Dourado I; Marcela Quintão Penha II; Luana Gischewski Campos II; Monize Ferreira Figueiredo de Carvalho I; Donaldo Rosa Pires Júnior III
I Programa de Pós-Graduação em Odontologia, Universidade Federal dos Vales do Jequitinhonha e Mucuri (UFVJM), Diamantina, MG, Brasil
II Departamento de Odontologia, Universidade Federal dos Vales do Jequitinhonha e Mucuri (UFVJM), Diamantina, MG, Brasil
III Departamento de Ciências Biológicas e da Saúde (FCBS), Universidade Federal dos Vales do Jequitinhonha e Mucuri (UFVJM), Diamantina, MG, Brasil
Contact: rafa18ms@hotmail.com, mauricio_mrd@hotmail.com, marcelaquintao@yahoo.com.br, luanagischewski@hotmail.com, monize_c@hotmail. com, donaldo@ufvjm.edu.br
ABSTRACT
Aim: The present study aimed to evaluate the in vitro antimicrobial activity of hydroalcoholic and aqueous extracts from leaves of P. guajava on four bacterial and two fungal samples to determine the range of minimum inhibitory concentration of the extracts. Material and Methods: The antimicrobial activity of extracts of different polarities was compared using the agar dilution method. Differences in susceptibility to the active ingredient could be observed. Results: The results showed that the hydroalcoholic extract was active against Escherichia coli (1024 μg/ml) and Bacillus cereus (900 μg/ml). The aqueous extract showed no antimicrobial activity. Conclusions: Despite demonstrating antimicrobial efficiency, the P. guajava extract may be insufficient or may require a higher concentration to be fully effective.
Uniterms: Psidium. Products with antimicrobial action. Traditional medicine.
RESUMO
Objetivo: Este estudo teve como objetivo avaliar a atividade antimicrobiana in vitro de extratos hidroalcoólico e aquoso das folhas de P. guajava em quatro amostras bacterianas e duas fúngicas para determinar o intervalo de concentração inibitória mínima dos extratos. Material e métodos: A atividade antimicrobiana de extratos com diferentes polaridades foi comparada através do método de diluição em ágar. Houve diferenças na susceptibilidade para o ingrediente ativo. Resultados: Os resultados mostraram que o extrato hidroalcoólico foi ativo contra Escherichia coli (1024 μg/ml) e Bacillus cereus (900 μg/ml). O extrato aquoso não mostrou atividade antimicrobiana. Conclusão: Apesar da demonstrada eficácia antimicrobiana, o extracto de goiaba pode não ser suficiente ou requer elevada concentração para ser eficaz.
Descritores: Psidium. Produtos com ação antimicrobiana. Medicina tradicional.
INTRODUCTION
In recent decades, the use of natural products for pharmacological purposes has witnessed a significant increase worldwide due mainly to attempts to minimize the side effects caused by synthetic drugs1. In addition, several microorganisms that are pathogenic to man have demonstrated resistance to commonly used antimicrobial agents, especially in the cases of cancer and immunosuppressed patients2. In this scenario, drug research is essential, and nature contains vast resources for this very purpose. By using folk knowledge, and by seeking scientific proof concerning this knowledge, it is possible to find substances that are both effective and less toxic to human health, and which are able to combat microorganisms that are commonly resistant to conventional drugs.3-10
In vitro studies using extracts from various plants showed an inhibitory action of microbial growth11. Among these, what stands out is the Psidium guajava, a fruit species of the Myrtaceae family, popularly known as the guava tree. This tree is widely distributed in all tropical and subtropical regions of the world and can be found throughout the Brazilian territory, attracting a wide range of research due to its biological activities12,13. The P. guajava L. leaves have been used throughout history in folk medicine, due to their anti-diarrheal, anti-microbial, anti-inflammatory, anti-spasmodic, antipyretic, and anti-mutagenic properties, to control hypertension, obesity and diabetes. Recently, research has demonstrated the inhibitory action of P. guajava L. leaves against gastric lesions and has shown their power to stimulate the healing of existing lesions. These leaves contain flavonoids, mainly quercetin derivatives, which are hydrolyzed in the body to manifest their various pharmacological actions15-17. In a study using an aqueous extract of guava leaves, antimicrobial activity was seen to combat against the Staphylococcus aureus and Streptococccus of groups mitis, oralis and mutans.18
The hydrophobic properties of the cell wall surface indirectly assist in the adherence of bacteria to the pellicle acquired from tooth surfaces. A study carried out with aqueous extracts of P. guajava leaves showed a reduction in the hydrophobic binding of the surface-cells of Streptococcus sanguis, Streptococcus mitis, and Actinomyces sp, indicating the positive benefits of this extract for dental care19. In folk medicine, the guava leaves have commonly been used to promote oral hygiene by destabilizing biofilms through mechanical actions, without understanding their antimicrobial properties.
Taking into account their widespread use by the general population and the therapeutic evidence of guava leaves, futher studies on the effectiveness of guava leaves are warranted in an attempt to verify both their action against pathogens and their stability, thus allowing for residual action. Therefore, the present study aims to evaluate the in vitro antimicrobial activity of Psidium guajava L. extracts against four bacterial strains and two fungal samples in order to obtain data on their ability to control microbial growth.
MATERIALS AND METHODS
Collection of plant sample
Leaves, flowers, and stems of the guava specimen were collected in the city of Diamantina (MG), Rua Cruz das Almas, number 460 / B, Barrio Bom Jesus, to make the exsiccate. The plant was identified as Psidium guajava L. (Myrtaceae), and the exsiccate was deposited in the DIAM herbarium at Universidade Federal dos Vales do Jequitinhonha e Mucuri (UFVJM), logged under protocol number 1432.
Leaves of the same specimen were collected at 6:00 am and sent to the Pharmacognosy and Phytochemistry Laboratory, Department of Pharmacy, UFVJM, for preparation of the extracts.
Preparation of the Extracts
Guava leaves were dried at room temperature and protected from light for approximately 10 days. To prepare the hydroalcoholic extract, 52.8427 g of dried leaves and deletions were used. The methodology used to prepare the extract was soaking. After the first soaking with ethanol (Synth®) and distilled water at a 3:1 ratio, over a nine-day period, the plant material was re-soaked with a new ethanol:water (3:1) solution for seven days. The hydroalcoholic extract was filtered and packaged in sealed glass vials and preserved under refrigeration until ready it was to be concentrated. The extract concentration was performed in a Rota Evaporator, model 802/ Fisatom®, at 40-45°C and at a reduced pressure to remove the ethanol. Subsequently, the extract was lyophilized in a model L101/Liotop® lyophilizer, resulting in a dried hydroalcoholic extract. To prepare the aqueous extract, 40.8625 g of dried leaves and deletions were used. After soaking in distilled water for seven days, the extract was filtered and placed in sealed glass vials, which were kept frozen. After, the aqueous extract was lyophilized, obtaining the dried aqueous extract.
Microoganisms
The present study used four reference bacterial samples from the culture collection of the Microbiology Laboratory of the Department of Basic Sciences (FCBS/UFVJM): Staphylococcus aureus ATCC29313, Bacillus cereus ATCC11778, Pseudomonas aeruginosa ATCC27853, and Escherichia coli ATCC25922, as well as two fungal samples: Saccharomyces cerevisiae and Candida sp., acquired in a local market and isolated from the oral cavity, respectively.
Samples Maintenance
Bacterial and fungal (Candida sp) samples were stored in a freezer (-18°C), in a storage medium of mineral salts and glycerin. The sample of Saccharomyces cerevisiae was maintained kept at a cool temperature until use. The Bacterial strains were grown in BHI broth (Brain Heart Infused - Micromed - Isofar ), and the yeast samples were grown in Sabouraud broth plus 2% sucrose (Micromed - Isofar ), incubated at 37°C for 12 hours in a bacteriological oven.
Susceptibility test
The susceptibility tests were performed by the agar dilution method (NCCLS, 1997) with the tested agents from the stock solutions being added to the culture medium at the time of the experiment. The concentrate of aqueous and hydroalcoholic extracts of guava leaves were suspended in dimethylsulfoxide - DMSO (VETEC) at 10 μg/mL to obtain the final concentrations of active principle between 4 and 1024 μg / ml. These diluted solutions were added to the culture medium to serve as a basis to determine the range of minimum inhibitory concentration (MIC) of the extracts. Figure 1 schematically illustrates the methodology used in this antimicrobial assay.
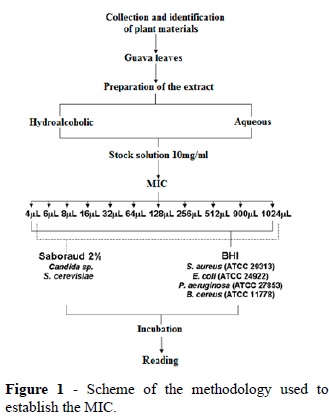
In experiments to determine the susceptibility profile, the inoculation of different microbial samples from the culture in broth and standardized to contain approximately 105 colony forming units (CFU) in specific media containing different concentrations of the antimicrobial agent was performed using a micropipette (Petpelm ) of variable volume 20-100 μl. The reading was made 24 hours after incubation for six samples. A control solid medium plus diluent DMSO was used. The MIC range was defined as that concentration in which no growth can be detected in the sample. For each condition at least two experiments were performed in duplicate.
RESULTS
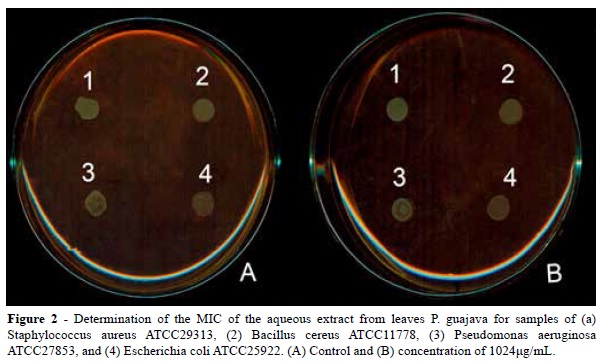
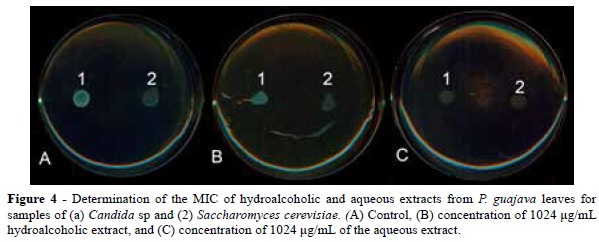
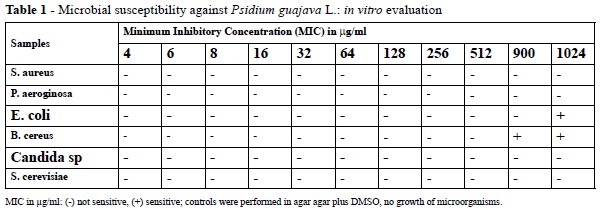
The results indicate, based on the tests performed on the solid medium, the aqueous extract of P. guajava presented no antimicrobial activity at the tested concentrations (4-1024 μg / mL) for any of the evaluated bacterial or fungal samples, as shown in Figures 2 and 4.
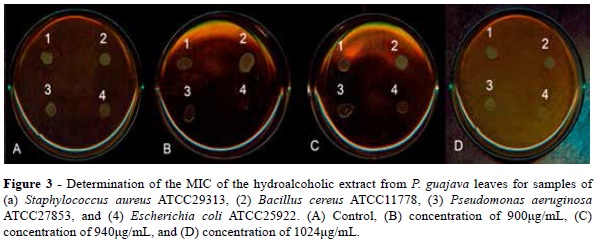
For the hydroalcoholic extract from P. guajava leaves, no inhibitory action could be not observed in the range from 4 to 1024 μg/ml for samples of S. aureus, P. aeruginosa, Candida sp, and S. cerevisiae. However, inhibitory activity could be observed in samples of B cereus (from 900 μg/ml) and E. coli (1024 μg/ml), as shown in Figures 3 and 4.
DISCUSSION
The present study indicates that the aqueous extract of P. guajava showed no antimicrobial activity on any of the microorganisms at any of the tested concentrations. By contrast, variations in the MIC values were found among the different microbial species for the hydroalcoholic extract. These differences in activity between the extracts can be explained by the probable variation in chemical composition, since they have different polarities, which can result in qualitative and quantitative differences of the active ingredients (such as tannins, for example, and other secondary metabolites). In another study, the antimicrobial potential of the aqueous extract of P. guajava against clinical isolates from multiresistant S. aureus produced an MIC of 7.5 mg/mL, which exceeded the limits tested in the present research21.
Another evaluation of the aqueous extract18 found positive results against to the reference strains of S. aureus, and strains of Streptococcus mitis and oralis isolated directly from the dogs' oral cavity. It is well-known that differences in susceptibility and variations in MIC values can be observed among the different microbial species for the same active ingredient, as well as between the different active ingredients for the same microbial species. Another study22 has shown that the hydroalcoholic and aqueous extracts of shoots of guava leaves proved to be active against S. aureus and E. coli. In this study, E. coli strains also showed sensitivity, but other results were not equivalent. The different results may be explained by the part of the plant used for extraction, given that young tissues and mature tissues differ with respect to chemical composition. By contrast, strains of E. coli and P. aeruginosa obtained from clinical infections showed no antimicrobial sensitivity to the hydroalcoholic extract from guava leaves.
Surveying the aqueous, methanol, and chloroform extracts of P. guajava, results showed an inhibition of 79 strains of S. aureus isolated from patients with respiratory infections. The lack of sensitivity found in this study may well be related to the fact that methanol and chloroform extracts are chemically different and may contain active ingredients that would not be present in the hydroalcoholic extract. The methanol extract of P. guajava was tested against standard cultures of E. coli, P. aeruginosa, and S. aureus, presenting an MIC of 500mg/mL for the former and of 250mg/mL for the latter two.23
Using the Mueller-Hinton broth microdilution technique, one study evaluated the antimicrobial activity of the hydroalcoholic extract of P. guajava. The MIC values found were of 500 μg/mL for E. coli, 1000 μg/mL for P. aeruginosa, 250 μg/mL for S. aureus, and 125 μg/mL for C albicans24 . In this present study, sensitivity was found only for E. coli, with MIC of 1024 μg/mL. The difference in results is justified by the technique of the diffusion/ dilution of microorganisms used in the studies and the difference in the extract composition: 90% ethanol and 10% water in the previously reported study and 75% ethanol and 25% water in the present study. Despite the growth inhibition of C. albicans within the hydroalcoholic extract, it was still impossible to identify the microbial species, which is a limitation of the study. Different species of the same microorganism can react differently to the P. guajava extracts.22
Currently, there are several methods to evaluate the antibacterial and antifungal activity of plant extracts. The antimicrobial activity of natural products is assessed by determining the MIC, considered as that in which no growth could be detected in any sample. The variations of this value depend on several factors, such as the technique applied, the microorganism and the type of strain used in the test, plant origin, harvest period, whether the extracts were prepared from fresh or dried plants, and the amount of extract tested. Thus, there is no standardized method for expressing the results of antimicrobial tests of natural products10. There are differences among the evaluated studies as regards the results, and variations could be observed between the results obtained in this study and the results found in the literature. Such variations may well be due to the different survey methods used, the different strains of microorganisms, and the different polarities of the evaluated extracts.
The in vitro antimicrobial efficiency can be interpreted as an indication of the therapeutic role. It should be noted, however, that in vitro data should not be directly extrapolated to the clinic, since in vitro tests do not consider several factors, such as variations in pH, antimicrobial substantivity, and microbiota composition, which can interfere in the clinical efficiency of these products.25,26
CONCLUSION
The results of this study demonstrated that the hydroalcoholic extract of P. guajava leaves, when used in high concentrations, can inhibit the growth of certain bacteria. These findings may provide the basis for new studies that seek to prove the efficiency of antimicrobial therapy against infections caused by these microorganisms.
REFERENCES
1. Malhotra N, Rao SP, Acharya S, Vasudev B. Comparative in vitro evaluation of efficacy of mouthrinses against Streptococcus mutans, Lactobacilli and Candida albicans. Oral Health Prev Dent. 2011; 9:261-8. [ Links ]
2. Reynolds E, Ross JI, Cove JH. Msr(A) and related macrolide/streptogramin resistance determinants: incomplete transporters? Int J Antimicrob Agents. 2003; 22:228-36.
3. Meng JC, Zhu QX, Than RX. New antimicrobial mono and sesquiterpenes from Soroseris hookeriana subsp. Erysimoides. Planta Med. 2000; 66, 541-4.
4. Ho KY, Tsai CC, Huang HS, Chen CP, Lin TC, Lin CC. Antimicrobial activity of tannin components from Vaccinium vitis-idaea L. J Pharm Pharmacol. 2001; 53, 187-91.
5. Michelin DC, Moreschi PE, Lima AC, Nascimento GGF, Paganelli MO, Chaud MV. Avaliação da atividade antimicrobiana de extratos vegetais. Rev Bras Farmacogn, 2005; 15: 316-20.
6. Leitão SG, Castro O, Fonseca EM, Julião LS, Tavares ES, Leo RRT, et al. Screening of Central and South American plant extracts for antimycobacterial activity by the Alamar Blue test. Rev Bras Farmacogn. 2006; 16: 6-11.
7. Lima IO, Oliveira RAG, Lima EO, Farias NMP, Souza EL. Atividade antifúngica de óleos essenciais sobre espécies de Candida. Rev Bras Farmacogn. 2006; 16: 197-201.
8. Barbosa-Filho JM, Nascimento-Júnior FA, Tomaz ACA, Athayde-Filho PF, Silva MS, Cunha EVL et al. Natural products with antileprotic activity. Rev Bras Farmacogn. 2007; 17:141-8.
9. Saúde-Guimarães DA, Faria AR. Substâncias da natureza com atividade anti-Trypanosoma cruzi. Rev Bras Farmacogn.2007; 17:455-65.
10. Ostrosky EA, Mizumoto MK, Lima MEL, Kaneko TM, Nishikawa SO, Freitas BR. Métodos para avaliação da atividade antimicrobiana e determinação da concentração mínima inibitória (CMI) de plantas medicinais. Rev Bras de Farmacogn. 2008; 18:301-7.
11. Betoni JEC, Mantovani RP, Barbosa LN, Stasi LCD, Junior AF. Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. Mem Inst Oswaldo Cruz. 2006; 101:387-90.
12. Gosh P, Mandal A, Chakraborty P, Rasul MG, Madhumita C, Saha A. Triterpenoids from Psidium guajava with Biocidal Activity. Indian J Pharmacol Sci. 2010; 72(4): 504-7.
13. Filho CC, Nachtigal JC, Kersten E. Propagação da goiabeira (Psidium guajava L.) pelo método de mergulhia de cepa. Rev Bras Agrocienc. 1995; 2:112-14.
14. Livingston RNR, Sundar K. Psidium guajava Linn confers gastro protective effects on rats. Eur Rev Med Pharmacol Sci. 2012; 16:151-6.
15. Dhiman A, Nanda A, Ahmad S, Narasimhan B. In vitro antimicrobial activity of methanolic leaf extract of Psidium guajava L. J Pharm Bioallied Sci.2011; 3: 226-9.
16. Metwally AM, Omar AA, Harraz FM, El Sohafy SM. Phytochemical investigation and antimicrobial activity of Psidium guajava L. leaves. Pharmacogn Mag. 2010; 6(23): 212-8.
17. Rattanachaikunsopon P, Phumkhachorn P. Bacteriostatic effect of flavonoids isolated from leaves of Psidium guajava on fish pathogens. Fitoterapia. 2007;78:434–6.
18. Menezes MC, Souza MMS, Botelho RP. In vitro evaluation of antimicrobial activity of brazilian plants extracts on bacteria isolated from oral cavity of dogs. Rev Univ Rural, Ser Cienc Vida. 2004; 24:141-4.
19. Razak FA, Othman RY, Rahim ZHA. The effect of Piper betle and Psidium guajava extracts on the cell-surface hydrophobicity of selected early settlers of dental plaque. J Oral Sci. 2006; 48:71- 5.
20. Anas K, Jayasree PR, Vijayakumar T, Manish- Kumar PR. In vitro antibacterial activity of Psidium guajava Linn. leaf extract on clinical isolates of multidrug resistant Staphylococcus aureus. Indian J Exp Biol.2008; 46:41-6.
21. Vieira RHSF, Rodrigues DP, Gonçalves FA, Menezes FGR, Aragão JS, Souza OV. Microbicidal effect of medicinal plant extracts (Psidium guajava Linn. and Carica papaya Linn) upon bacteria isolated from fish muscle and know to induce diarrhea in children. Rev Inst Med Trop 2001; 43:145-8.
22. Gonçalves AL, Alves Filho A, Menezes H. Estudo comparativo da atividade antimicrobiana de extratos de algumas árvores nativas. Arq Inst Biol. 2005; 72:353-8.
23. Cheruiyot KR, Olila D, Kateregga J. In-vitro antibacterial activity of selected medicinal plants from Longisa region of Bomet district, Kenya. Afr Health Sci. 2009 August; 9: 42–6.
24. Holetz FB, Pessini GL, Sanches NR, Cortez DAG, Nakamura CV, Filho BPD. Screening of Some Plants Used in the Brazilian Folk Medicine for the Treatment of Infectious Diseases. Mem Inst Oswaldo Cruz. 2002; 97:1027-31.
25. Marsh PD. Microbiological aspects of the chimical control of plaque and gingivitis. J Dent Res. 1992; 71:1431-8.
26. Fernandes TG, Mesquita ARC, Randau KP, Franchitti AA, Ximenes EA. In vitro synergistic effect of Psidium guineense (swartz) in combination with antimicrobial agents against methicillin-resistant Staphylococcus aureus strains. Scientific World Journal. 2012.













