Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Arquivos em Odontologia
versão impressa ISSN 1516-0939
Arq. Odontol. vol.51 no.4 Belo Horizonte Out./Dez. 2015
Lack of association between IL1A c.-889 C>T gene polymorphism and chronic periodontitis in subjects from Bahia, Brazil
Ausência de associação entre o polimorfismo gênico IL1A c.-889 C>T e a periodontite crônica em indivíduos da Bahia, Brasil
Kamilla Menezes Oliveira* I; Lucas Magalhães Alcântara* II; Fernanda Gabriela Teixeira I; Samir Andrade Mendonça I; Djanilson Barbosa dos Santos II; Lucas Miranda Marques III; Maise Mendonça Amorim IV; Raquel de Souza Gestinari V
* These authors contributed equally to the work presented in this article
I Laboratory of Cellular and Molecular Biology, Multidisciplinary Health Institute, Federal University of Bahia (UFBA), Vitória da Conquista, BA, Brazil
II Health Sciences Center, UFBA Recôncavo, Santo Antonio de Jesus, BA, Brazil
III Laboratory of Microbiology and Immunology, Multidisciplinary Health Institute, Federal University of Bahia, Vitória da Conquista, BA, Brazil
IV Laboratory of Cellular and Molecular Biology, Multidisciplinary Health Institute, Federal University of Bahia, Vitória da Conquista, BA, Brazil
V Núcleo em Ecologia e Desenvolvimento Socioambiental de Macaé (NUPEM), Federal University of Rio de Janeiro (UFRJ), Macaé, RJ, Brazil
Contatos: kmillamo@gmail.com, lucas.biotec@hotmail.com, fernanda.gab.teixeira@gmail.com, samir.446@gmail.com, dejab@bol.com.br, lmirandamarques@gmail.com, maimendonca@hotmail.com, raquelgestinari@gmail.com
ABSTRACT
Aim: This study aimed to analyze the relationship between the polymorphism c.-889 C>T in the IL1A gene and periodontitis in subjects from Vitória da Conquista, Bahia, Brazil. Methods: Two hundred fifty-nine individuals were classified according to periodontitis diagnoses in cases and controls. Genomic DNA was extracted from buccal epithelial cells. Genotyping was performed by PCR-RFLP, followed by agarose gel electrophoresis. Data were analyzed using Chi-square, Fisher's exact and Hosmer-Lemeshow tests. Results: In the individuals evaluated in this study, periodontitis was significantly associated with a low educational level (p = 0.024) and clinical variables, such as gingival bleeding (p = 0.019) and tooth mobility (p = 0.001). Among patients in the case group, the majority (72.88%) had a generalized form of periodontitis. The genotypic frequencies for IL1A c.–889 C>T between the case and control groups did not significantly differ (χ2 = 1.6983; p = 0.428). Conclusion: This study's findings indicate the lack of association between periodontitis and the IL1A c -889 C>T gene polymorphism.
Uniterms: Peridontitis. Genetic polymorphism. Interleukin-1.
RESUMO
Objetivo: Este estudo teve por objetivo analisar a relação entre o polimorfismo c.-889 C> T no gene IL1A e periodontite em usuários do Sistema Único de Saúde de Vitória da Conquista, Bahia - Brasil. Material e Métodos: Duzentos e cinquenta e nove indivíduos foram classificados quanto ao diagnóstico de periodontite em casos e controles. O DNA genômico foi extraído a partir de células epiteliais bucais. A genotipagem foi realizada por PCR-RFLP, seguida pela eletroforese em gel de agarose. Os dados foram analisados por meio dos testes de Quiquadrado, exato de Fisher e Hosmer-Lemeshow. Resultados: Entre os indivíduos analisados, a periodontite mostrou-se significativamente associada ao menor grau de escolaridade (p = 0,024) e às variáveis clínicas sangramento gengival (p = 0,019) e mobilidade dentária (p = 0,001). Dentre os pacientes do grupo caso, a maioria (72,88%) apresentou a forma generalizada da periodontite. As frequências genotípicas para o polimorfismo IL1A c. 889-C> T entre os grupos caso e controle não diferiram significativamente (χ2 = 1.6983; p = 0,428). Conclusão: Nossos achados indicam a ausência de associação entre a periodontite e o polimorfismo no gene IL1A c -889 C> T na amostra avaliada.
Descritores: Periodontite. Polimorfismo genético. Interleucina-1.
INTRODUCTION
Periodontitis is a chronic inflammatory disease associated with the destruction of tooth supporting tissues. This disease results from an inflammatory cascade generated by the interaction between periodontopathogenic bacteria and the host immune response, which includes a complex network of cytokines, reactive oxygen species, and proteolytic enzymes1,2.
The differentiated response to bacterial pathogenicity has been described as the basis for the individual susceptibility to periodontitis. Modulating mechanisms of the host immune response to periodontal pathogens are seen as essential in disease development and progression, which varies among affected patients2,3. Hence, it is indeed important to understand the etiologic and pathogenic mechanisms that trigger aggressive and destructive responses in tissues, thus fostering the development of more effective diagnostic and therapeutic approaches to periodontal disease.
Although pathogenic microbiota and environmental risk factors are considered critical factors involved in the pathogenesis of periodontitis, evidence also points to an important role of the genetic component, which associates multigenic predisposition with disease etiology and characterizes periodontitis as a disease of complex inheritance4. Studies that have evaluated the role of genetic factors in the immune response in periodontitis suggest that polymorphisms in genes that codify cytokines may cause structural and/or functional changes in the encoded protein or in their expression levels. Thus, it is proposed that the severity and/or progression of periodontitis may be related to these allelic variants, which partly explains the differences observed in the individual immune response against periodontopathogenic bacteria5.
The interaction of pro- and anti-inflammatory cytokines has been described as a critical step in the pathogenesis of periodontitis1-3. In recent years, there has been a remarkable increase in the understanding of the role of the cytokines produced in bone environments. Although several immune mediators are related to the development of the tissue inflammatory response, interleukins 1 (IL-1α and β), the tumor necrosis factor α (TNF-α), lymphotoxin (TNF-β), and interleukin-6 (IL-6) have been considered to be the most important cytokines involved in inflammation-related periodontitis6. In particular, IL-1 interleukins have the capacity to stimulate bone resorption and may regulate fibroblast proliferation, both in the gingival tissue and in the periodontal ligament, which is considered critical for resolution of the patient's clinical condition7.
Several genetic polymorphisms have been described in the genes of IL-1 clusters and were associated with alterations in this protein expression and, consequently, in the pathophysiology of periodontitis8,9. The polymorphism that results from cytosine for thymine switching in a -889 position (c.-889C>T - rs1800587) in the IL1A gene is among these. Nevertheless, conflicting results regarding the association of this polymorphism with periodontitis in different population samples have been reported4,10-12. These findings corroborate the importance of the analysis of this variant in other populations so as to better elucidate the role of this polymorphism within periodontitis. Therefore, the aim of the present study was to investigate the relationship between the IL1A c.-889 C>T polymorphism and periodontitis in a sample from a study conducted in Vitória da Conquista, Bahia, Brazil.
METHODS
Ethical aspects
Ethical approval for this research was obtained from the Research Ethics Committee of the State University of Southwest of Bahia. All experiments were conducted in accordance with the Declaration of Helsinki, and written consent forms were signed by all participants who agreed to participate in the study. The project's data collection process was also approved and authorized by the Health Department of Vitória da Conquista. Casuistic The sample population of the present study is characteristically multiethnic, consisting of 259 unrelated individuals of both genders (52 males and 207 females), between 25 and 69 years of age, who received medical care at public health units of the National Health System of Vitória da Conquista, Bahia, Brazil. This urban area of this city contained 23 health units, of which 10 offered dental care from December 2009 to February 2011, period in which clinical examinations were performed and materials were collected. To analyze the demographic data and clinical aspects of periodontitis, anamnesis was performed. Individuals were excluded from the study if they (i) were pregnant or breastfeeding, edentulous, or children; (ii) had received antibiotic treatment in the previous 3 months; (iii) were taking long-term anti-inflammatory or immunosuppressive drugs and/ or (iv) were diagnosed with aggressive periodontitis. Based on these criteria, patients were divided into two distinct groups, 127 chronic periodontitis patients (Age: 25-69 years/ 40.12 ± 13.09 - mean ± standard deviation) and 132 healthy controls (Age: 25-62 years/ 38.80 ± 11.49 - mean ± standard deviation). The sample size was calculated considering a 5% significance level based on the expected prevalence of 40%13 and a desired accuracy of 4.0%, using the STATA 12 program (Stata Corp., College Station, USA). The minimum sample size was 250 individuals. The sample consisted of users of all public health units who had received dental care.
Clinical examination
The clinical examination was performed by the dentists of the public health units, who had been properly trained to diagnose periodontitis. The diagnosis was based on: 1) the presence of four teeth with one or more sites that presented deep probing ≥4 mm and 2) clinical attachment loss (CAL) ≥ 3 mm and bleeding on probing in the same site13. Diagnostic criteria were evaluated as follows: 1) probing pocket depth (PPD) - calculated by distance in millimeters from the free gingival margin to the bottom of the pocket; 2) gingival recession - represented by the distance between the cement-enamel junction (CEJ) and the gingival margin; 3) CAL - calculated by the distance in millimeters from the CEJ to the bottom of the pocket; 4) During the measurement of PPD, when the gingival margin was more coronal in relation to the CEJ, the distance between the two, used to calculate the CAL, proved to be smaller14.
Collection of biological material
The sampling was made by scraping the oral mucosa using a custom-made sterile wooden spatula, which was then immediately immersed in a 2mL sterile microtube. The samples were stored at -20°C until their use for molecular analysis.
DNA extraction and genotyping
DNA extraction was performed by applying the alkaline technique according to the protocol described by Saab et al.15.
Genomic DNA was amplified by Polymerase Chain Reaction - Restriction Fragment Length Polymorphism (PCR-RFLP), as described in prior literature16. The amplicon consisted of 99pb, considering the following oligonucleotides: 5′-AAGCTTGTTCTACCACCTGAACTAGGC-3′/ 5′-TTACATATGAGCCTTCCATG-3′. PCR reactions were performed in 25 uL and included 20 pM of primers and 1 U of Taq DNA polymerase (Invitrogen Life Technologies®, São Paulo, Brazil). Thermal cycling conditions included an initial denaturation at 94°C for 10 min, followed by 45 cycles of amplification at 94ºC for 1min, and annealing at 50°C for 1 min 20s, with an extension for 1 min 20s at 72°C, and with a final extension for 5 min at 72°C.
The amplified fragments were digested with the restriction enzyme Nco I16 following the manufacturer's protocol (New England BioLabs Inc, Ipswich, MA, USA). The digestion product was analyzed by horizontal electrophoresis on a 4.0% agarose gel in 1X TBE buffer stained with ethidium bromide (0.5 mg/mL) and viewed in a UV light transilluminator (LABImage L-PIX (H.E.) - Loccus Biotecnologia, Cotia, SP,Brazil).
Statistical analyses
The magnitude of the associations between risk factors and periodontitis was estimated by the odds ratio (OR) with a 95% confidence interval (95% CI). The Chi-square test (χ2) measured the association between the independent (age, gender, educational level, smoking, tooth mobility, gingival bleeding, dentin sensitivity, and discomfort when chewing) and dependent (periodontitis) variables. All variables with p < 0.20 in the χ2 were included in the multivariate analysis using logistic regression. A backward deletion strategy was applied, and those variables with p-values of equal to or less than 0.05 remained in each of the final models. Goodness of fit was evaluated using the Hosmer-Lemeshow test, while the Stata software version 12.0 was used for data analysis (Stata Corp., College Station, USA).
Allele and genotype frequencies were obtained by direct counting. Comparison between the groups was made using the Chi-square and Fisher's exact tests for other categorical variables. Associations with a p-value < 0.05 were considered statistically significant.
RESULTS
In the present study, 259 subjects from Vitória da Conquista, Bahia, Brazil were evaluated regarding periodontal health, demographic profile, and most common signs and symptoms for periodontitis. Table 1 presents the basic characteristics of the selected subjects, as well as the general results of the periodontal examination and anamnesis. The data obtained in the bivariate analysis suggest that there is a positive relationship between periodontitis and low educational level (χ2 = 5.2364, p = 0.024), tooth mobility (χ2 = 10.5720, p = 0.001), and gingival bleeding (χ2 = 5.6007, p= 0.019). Smoking revealed an association with periodontitis, but this result proved to be statistically insignificant (OR= 1.96-95% CI= 0.99-3.84/ p = 0.051). The remaining variables were not statistically associated with the disease.
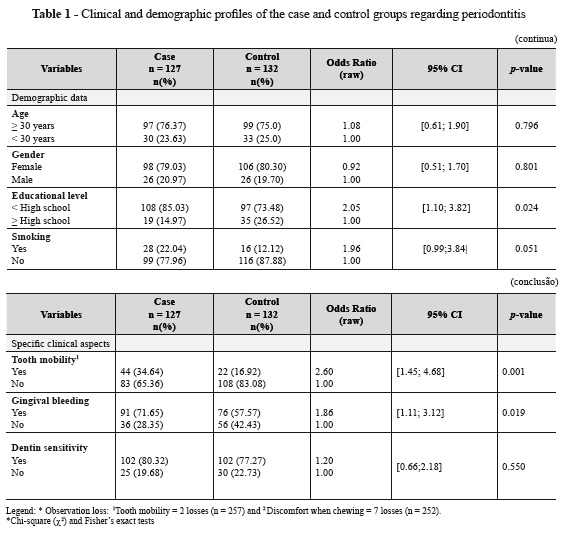
Table 2 presents the case group's data regarding the variables of probing depth, number of teeth, and affected sites. Among these individuals, 27.12% had up to 30% of the teeth affected by periodontitis, whereas 23.73% presented 31-60%, 11.86% presented Legend: * Observation loss: 1Tooth mobility = 2 losses (n = 257) and 2 Discomfort when chewing = 7 losses (n = 252). *Chi-square (χ2) and Fisher's exact tests Variables Case n = 127 n(%) Control n = 132 n(%) Odds Ratio (raw) 95% CI p-value Specific clinical aspects Tooth mobility1 Yes No 44 (34.64) 83 (65.36) 22 (16.92) 108 (83.08) 2.60 1.00 [1.45; 4.68] 0.001 Gingival bleeding Yes No 91 (71.65) 36 (28.35) 76 (57.57) 56 (42.43) 1.86 1.00 [1.11; 3.12] 0.019 Dentin sensitivity Yes No 102 (80.32) 25 (19.68) 102 (77.27) 30 (22.73) 1.20 1.00 [0.66;2.18] 0.550 (conclusão) 61-80%, and 37.29% presented 81-100% of the teeth affected by periodontitis. This group also showed that 27.12% of the patients had localized periodontitis (less than 30% of affected sites), while 72.88% presented widespread periodontitis (over 30% of affected sites).
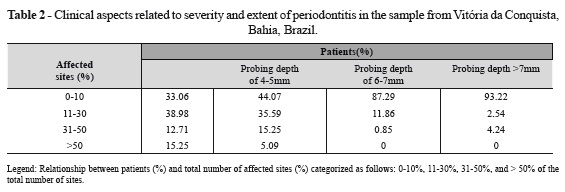
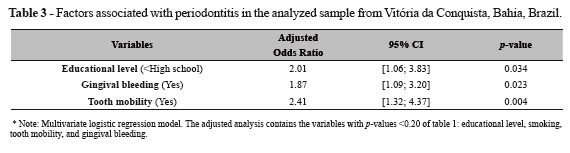
Table 3 presents the odds ratios (OR) for periodontitis with their respective confidence intervals (95% CI) estimated by the multivariate regression model. The results indicate a positive association between periodontitis and the variables of educational level (High school), gingival bleeding, and tooth mobility.
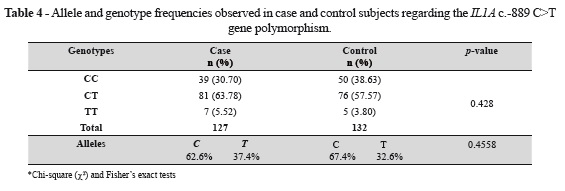
The selected sample was also evaluated for the relationship between the IL1A c.-889 C>T gene polymorphism and periodontitis. The results are shown in table 4. Genotype frequencies obtained in the total population were 34.7% for the CC genotype, 60.6% for the TC genotype, and 4.7% for the TT genotype. The frequency of the C allele in the population was 65.1%, and the T allele was 34.9%. The observed genotype frequencies did not significantly differ between the analyzed case and control groups (χ2 = 1.6983; p = 0.428), thus making it impossible to establish a relationship between the polymorphism IL1A c.-889 C>T and periodontitis.

DISCUSSION
The periodontitis etiological factors evaluation has been pinpointed as an important tool for the development and implementation of public health system policies, aimed at reducing the prevalence of periodontitis and other oral diseases in different populations17. In the studied population, among the assessed demographic aspects, the lowest educational level proved to be significantly associated with periodontitis. Nevertheless, for the variables of gender and age, no significant association was observed. Age, education, socioeconomic status and smoking have been described as potential risk factors for periodontitis in several populations. Associations of disease with obesity, hormonal changes, cardiovascular disease, and pregnancy have also been researched18.
Regarding age, although it has been identified as a risk factor for periodontitis by several authors, it is understood that this disease should not be considered a natural event of aging, and that this association is a result of the cumulative nature of periodontitis19,20. No association between the age risk factor and periodontitis was observed in this study's population. Nevertheless, results of periodontal disease prevalence and/or severity from different studies show that this disease is more common in older-aged groups when compared to younger-aged groups19. On the other hand, it is still unclear whether aging per se is a risk factor for severe periodontitis, or if its effect is due to the prolonged exposure of older subjects to other etiologic factors.
Periodontal disease also presents a reciprocal relationship with educational level. Several studies have pointed out that this association is directly related to low educational levels17,21. Likewise, in the present study, the risk factor of education level was significantly associated with periodontitis, since the disease was most frequently observed among individuals who have not completed high school. Another study conducted in a Brazilian population revealed that the risk of dental insertion loss due to disease progression was increased by 53% in individuals who studied four years or less, compared to those who have completed the graduation course19. Similarly, Buchwald et al.22 showed that the prevalence of periodontitis in a German population sample was related to socioeconomic status, and educational level proved to be the determining factor for the disease.
The effect of smoking on general health has been widely discussed in the medical literature. It is known that smoking is closely linked to an increased risk of heart disease, various cancers, and lung diseases. The data found in this sample showed an association, although not statistically significant (p = 0.051), between smoking and periodontitis, which is similar to studies that link smoking as one of the most prevalent risk factors for periodontitis. These studies indicate that smoking exerts a substantial destructive effect on the periodontal tissues and increases the rate of periodontal disease progression17. Furthermore, these studies suggest that 40% of the cases of chronic periodontitis are referent to smokers, and reported that these individuals have a 2-7 times higher risk of developing periodontal disease and dental insertion loss when compared to nonsmokers23. In another study conducted in Brazil to assess the effects of smoking on periodontitis, it was observed that smokers presented a significantly higher mean probing depth and level of dental insertion loss when compared to nonsmokers20.
The exacerbation of the inflammatory processes in response to the stimulus generated by pathogenic bacteria in the oral cavity results in the progressive destruction of the supporting tissues, which is manifested in characteristic signs: dentin sensitivity, gingival bleeding, increased tooth mobility, and discomfort when chewing24. In the present study, statistically significant associations with periodontitis were observed regarding tooth mobility and gingival bleeding. However, dentin sensitivity and discomfort when chewing were not significantly associated with periodontitis. These clinical variables are not considered pathognomonic clinical signs of periodontitis, which can be seen in other diseases that affect the oral cavity, such as gingivitis. Thus, it is possible that patients who are not diagnosed with periodontitis have presented dentin sensitivity and/or discomfort when chewing, since these variables did not statistically show significant differences among the groups25,26.
Sequelae of periodontal destruction may be assessed and reported at subject, tooth, and site levels. A common approach to describe the periodontal status of a sample or population is to report the individual's average periodontal probing depth. Alternatively, the periodontal status may be described by reporting the prevalence and extent of clinical parameters according to the severity of the disease. In this context, prevalence is defined as the percentage/number of individuals presenting the condition. Severity is provided by the clinical use parameters27.
Higher periodontitis estimates among adults were also found in Porto Alegre, Brazil. Among subjects over 18 years of age presenting at least six teeth, 59.9% of the subjects showed periodontal probing depth ≥ 5 mm with 14.0% of the teeth affected28. Some similar findings were observed in a convenience sample of adults who received dental care at a public Dental School in Rio de Janeiro29. Another study examined a large sample of patients seeking dental care in private practices, hospitals, and community health centers in 23 different regions of Argentina. Overall, the prevalence of periodontal probing depth of 3.5 - 5.5 mm and ≥ 5.5 mm were 26.4% and 14.3%, respectively30. In general, studies on the prevalence of destructive periodontal disease in urban and isolated areas of Latin America indicate a high prevalence and low extent of moderate to severe CAL27.
The subjects in the present work showed clinical parameters that allowed them to be characterized according to the severity and extent of the disease. Regarding the probing depths, 35.59% of the patient population showed 11% to 30% of the sites with probing depths ranging from 4 mm to 5 mm; however, the pocket depths of greater than 6 mm were observed in 14.40% of the sample. These data demonstrate that although a significant portion of the sample presented probing depths of greater than 4 mm, most ranged between 4mm and 5mm. In 49.15% of the population, the teeth affected by the disease ranged from 60% to 100%, which is an extensive involvement in nearly half of the complete sample. The disease severity in the population can also be exemplified by the percentage of patients presenting: 11% to 30% of affected sites, 38.98% of the sample; over 31% of affected sites, 27.6% of the sample; 31% to 50% of affected sites, 12.71% of the sample; and over 50% of affected sites, 15.25%. Regarding the disease localization, 72.88% presented widespread disease, which expresses the extension of periodontitis in these individuals. These data reflect the severity and significant extension of periodontitis in the study sample, which clearly requires effective intervention of public health policies in order to reduce the prevalence and severity of the disease and its consequences.
Although infection by pathogens is essential for the onset of periodontitis, their presence and/or amount in the oral cavity is insufficient to explain the differences among individuals regarding the severity of the disease8. Such characteristics would be mostly related to the genetic profile of the individuals, especially those related to the expression of gene coding molecules involved in the host immune response in periodontitis31. Although several immune mediators influence the development of the inflammatory response in the tissue, IL-1 genes are considered to be the main cytokines involved in most inflammatory responses6. IL-1α and IL-1β have pro-inflammatory properties and can be found in increased levels in periodontal disease. IL-1 is also considered a key regulator of the host responses to microbial infection and a major modulator of extracellular matrix catabolism and bone resorption31. Additionally, polymorphisms in the IL-1 genes have a direct functional significance by altering the gene transcription or the protein production. These have also been connected with other complex diseases32.
Several studies have attempted to relate the IL1A c.-889 C> T gene polymorphism and the etiology of periodontal disease. The results obtained in this study point to the lack of association between this polymorphism and periodontitis in the sample from Northeastern Brazil. Braosi et al.10 examined a possible association between IL1A c.-889 C> T gene polymorphism and periodontal disease in a population sample of 246 individuals from Southern Brazil. Although the level of transcription of the IL1A gene was increased in the presence of the T allele, genotype frequencies did not differ significantly between the evaluated case and control groups. These findings are in accordance with those obtained in the present study, in which the association between the polymorphism and periodontitis was also insignificant.
In contrast, studies conducted on multiethnic population-based samples from the Brazilian Southeast and on Caucasians of German origin revealed a statistically significant association between periodontitis and the -889 polymorphism. In these studies, the T allele was considered a risk factor for the disease11,33. Similarly, according to a meta-analysis performed by Nikolopoulos et al.4 involving eight studies, mostly conducted on Caucasian population samples, the T allele for the polymorphism in question was also more frequent in periodontitis cases when compared to controls. The results of the meta-analysis indicated a moderate positive association between IL1A c.-889 C> T gene polymorphism and chronic periodontitis in Caucasians. Recently, two other meta-analyses revealed similar results and showed a positive association between the -889 polymorphism and chronic periodontitis in population samples of Caucasian and Asian origin34,35. These results differ from those observed in the current study.
The analysis of the relationship between the IL1A c.-889 C> T gene polymorphism and periodontitis also showed that the frequency of the T allele varies from population to population, but has generally been lower in Asian populations. The allele and genotype frequencies observed for the population analyzed in this study, which is characteristically multiethnic, resemble those observed in previously analyzed populations4. These data demonstrate that allele frequencies can vary between different ethnic groups, enabling the observation of positive associations between a genetic polymorphism and a particular disease in a given population, and that these cannot necessarily be extrapolated to other populations 5. The present study did confront some limitations that should be considered. First, the results obtained concerning IL1A c.-889 C>T gene polymorphism can still be added to the analysis of other factors related to the etiology of periodontitis not evaluated here, including other genetic markers. Second, the results of the present study would have been more authentic if quantitative detection of IL- 1α cytokine levels had been applied to both the case and control groups. However, due to the higher cost of ELISA kits used for this purpose, IL-1α cytokine levels were not evaluated. Third, clinical examinations were performed by different professionals in the health units; therefore the possibility of minor discrepancies in diagnostic procedures among patients cannot be excluded.
Therefore, it can be concluded that, in this work, no association could be observed between the IL1A c.-889 C>T gene polymorphism and periodontitis in individuals from Vitória da Conquista, Bahia, Brazil. This result can be explained by the fact that periodontitis is a disease of complex inheritance, which is determined by the interaction of environmental and genetic factors. Thus, it is possible that other factors, not only environmental, but also other genetic factors not assessed in this study, may well have caused periodontitis in patients from the present sample. However, it was possible to confirm the statistically significant association between periodontitis and other factors, such as educational level (<High school), tooth mobility, and gingival bleeding, in the investigated subjects.
ACKNOWLEDGEMENTS
The authors would like to thank the patients that participated in this study and the dentists from the health public units in Vitória da Conquista, Bahia, Brazil, who performed the patients' clinical examinations. No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Taylor JJ, Preshaw PM, Donaldson PT. Cytokine gene polymorphism and immunoregulation in periodontal disease. Periodontol 2000 2004; 35: 158-82. [ Links ]
2. Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010; 89: 1349-63.
3. Feghali K, Tanabe S, Grenier D. Soluble CD14 induces cytokine release by human oral epithelial cells. J Periodontal Res. 2011; 46: 147-52.
4. Nikolopoulos GK, Dimou NL, Hamodrakas SJ, Bagos PG. Cytokine gene polymorphisms in periodontal disease: a meta-analysis of 53 studies including 4178 cases and 4590 controls. J Clin Periodontol. 2008; 35: 754-67.
5. Laine ML, Loos BG, Crielaard W. Gene polymorphisms in chronic periodontitis. Int J Dent. 2010; 324719.
6. Nicklin MJ, Barton JL, Nguyen M, FitzGerald MG, Duff GW, Kornman K. A sequence-based map of the nine genes of the human interleukin-1 cluster. Genomics 2002; 79: 718-25.
7. Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003; 74: 391-401.
8. Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000 1997; 14: 33-53.
9. Fiebig A, Jepsen S, Loos BG, Scholz C, Schafer C, Ruhling A, et al. Polymorphisms in the interleukin-1 (IL1) gene cluster are not associated with aggressive periodontitis in a large Caucasian population. Genomics 2008; 92: 309-15.
10. Braosi AP, Souza CM, Luczyszyn SM, Dirschnabel AJ, Claudino M, Olandoski M, et al. Analysis of IL-1 gene polymorphisms and transcript levels in periodontal and chronic kidney disease. Cytokine 2012; 60: 76-82.
11. Moreira PR, Costa JE., Gomez RS, Gollob KJ, Dutra WO. The IL1A (-889) gene polymorphism is associated with chronic periodontal disease in a sample of Brazilian individuals. J Periodont Res. 2007; 42: 23–30.
12. Trevilatto PC, Souza Pardo AP, Scarel-Caminaga RM, de Brito RB, Jr., Alvim-Pereira F, Alvim- Pereira CC, et al. Association of IL1 gene polymorphisms with chronic periodontitis in Brazilians. Arch Oral Biol. 2011; 56: 54-62.
13. Gomes-Filho IS, Sarmento VA, Rosing CK, Viana MIP, Trindade SC, Freitas COT, et al. Critérios para o diagnóstico clínico da doença periodontal. JBC Odontol Integr. 2005; 9:88-89.
14. Stecca L, Souza AA, Khan S. Influência da seleção de sítios periodontais específicos e da utilização de diferentes níveis de perda de inserção clínica na determinação da prevalência e severidade da periodontite. Periodontia 2009; 19:94-103.
15. Saab YB, Kabbara W, Chbib C, Gard PR. Buccal cell DNA extraction: yield, purity, and cost: a comparison of two methods. Genet Test. 2007; 11: 413-16.
16. Brett PM, Zygogianni P, Griffiths GS, Tomaz M, Parkar M, D'Aiuto F, et al. Functional gene polymorphisms in aggressive and chronic periodontitis. J Dent Res. 2005; 84: 1149-53.
17. Yousef A. AlJehani. Risk factors of periodontal disease: review of the literature. Int J Dent. 2014; 2014.
18. Oppermann RV, Weidlich P, Musskopf ML. Periodontal disease and systemic complications. Braz Oral Res. 2012; 26, Suppl 1:39-47.
19. Haas AN, Wagner MC, Oppermann RV, Rosing CK, Albandar JM, Susin C. Risk factors for the progression of periodontal attachment loss: a 5-year population-based study in South Brazil. J Clin Periodontol. 2013; 41: 215-23.
20. Ragghianti MS, Greghi SL, Lauris JR, Sant'ana AC, Passanezi E. Influence of age, sex, plaque and smoking on periodontal conditions in a population from Bauru, Brazil. J Appl Oral Sci. 2004; 12: 273-79.
21. Thomson WM, Shearer DM, Broadbent JM, Foster Page LA, Poulton R. The natural history of periodontal attachment loss during the third and fourth decades of life. J Clin Periodontol. 2013; 40: 672-80.
22. Buchwald S, Kocher T, Biffar R, Harb A, Holtfreter B, Meisel P. Tooth loss and periodontitis by socio-economic status and inflammation in a longitudinal population-based study. J Clin Periodontol. 2013; 40: 203-11.
23. Bergström J. Tobacco smoking and risk for periodontal disease. J Clin Periodontol. 2003; 30: 107-13.
24. Lopez R, Frydenberg M, Baelum V. Clinical features of early periodontitis. J Periodontol. 2009; 80: 749-58.
25. Newbrun E. Indices to measure gingival bleeding. J Periodontol 1996; 67: 555-61.
26. Liu H, Marcus M, Maida CA, Wang Y, Shen J, Spolsky VW. Predictive power of the severity measure of attachment loss for periodontal care need. J Periodontol. 2013; 84: 1409-15.
27. Oppermann RV, Haas AN, Rösing CK, Susin C. Epidemiology of periodontal diseases in adults from Latin America. Periodontol. 2000 2015; 67:13-33.
28. Susin C, Haas AN, Valle PM, Oppermann RV, Albandar JM. Prevalence and risk indicators for chronic periodontitis in adolescents and young adults in south Brazil. J Clin Periodontol 2005; 76: 262-67.
29. Silva-Boghossian CM, Luiz RR, Colombo AP. Periodontal status, sociodemographic and behavioral indicators in subjects attending a public dental school in Brazil: analysis of clinical attachment loss. J Periodontol 2009; 80: 1945-54.
30. Romanelli H, Gonzalez y Rivas M, Chiappe V, Gomez M, Macchi R. Periodontal treatment needs in Argentine adult subjects, Acta Odontol Latinoam 2007; 20: 39-47.
31. McDevitt MJ, Wang HY, Knobelman C, Newman MG, di Giovine FS, Timms J, et al. Interleukin-1 genetic association with periodontitis in clinical practice. J Periodontol. 2000; 71: 156-63.
32. Shapira L, Wilensky A, Kinane DF. Effect of genetic variability on the inflammatory response to periodontal infection. J Clin Periodontol. 2005; 32 Suppl 6: 72-86.
33. Wagner J, Kaminski WE, Aslanidis C, Moder D, Hiller KA, Christgau M, et al. Prevalence of OPG and IL-1 gene polymorphisms in chronic periodontitis. J Clin Periodontol. 2007; 34: 823- 27.
34. Karimbux NY, Saraiya VM, Elangovan S, Allareddy V, Kinnunen T, Kornman KS, et al. Interleukin-1 gene polymorphisms and chronic periodontitis in adult whites: a systematic review and meta-analysis. J Periodontol. 2012; 83: 1407- 19.
35. Mao M, Zeng XT, Ma T, He W, Zhang C, Zhou J. Interleukin-1alpha -899 (+4845) C-- >T polymorphism increases the risk of chronic periodontitis: evidence from a meta-analysis of 23 case-control studies. Gene 2013; 532: 114-19.













