Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Stomatos
versão impressa ISSN 1519-4442
Stomatos vol.17 no.33 Canoas Jul./Dez. 2011
SCIENTIFIC ARTICLE
Effect of carbamide peroxide and neutral fluoride on enamel surface
Efeito do peróxido de carbamida e do flúor neutro sobre a superfície do esmalte
Roberta de Azambuja Tagliari1; Eduardo Galia Reston2; Alcebiades Nunes Barbosa3; Ricardo Prates Macedo4
1MSc candidate, Universidade Luterana do Brasil (ULBRA), Canoas, RS, Brazil.
2PhD in Cosmetic Dentistry – UNESP, Brazil. Professor, Faculty of Dentistry, ULBRA, Canoas, RS, Brazil.
3PhD in Cosmetic Dentistry – FOB, Brazil. Professor, Faculty of Dentistry, ULBRA, Canoas, RS, Brazil.
4PhD in Cosmetic Dentistry – UNESP, Brazil. Professor, Faculty of Dentistry, ULBRA, Canoas, RS, Brazil.
ABSTRACT
This study evaluated the effect of a 10% carbamide peroxide gel on enamel surface and the remineralizing capacity of neutral fluoride applied either during or after bleaching treatment. Sixteen fragments were obtained from four extracted human teeth (incisors and molars) and assigned to four groups. Group I served as the control and groups II, III and IV underwent daily 8-hour carbamide peroxide bleaching treatment for 2 weeks. Additionally, group III was treated with 2% neutral fluoride gel for 4 minutes/day, and group IV received one single application of fluoride gel at the end of the 2-week bleaching treatment. Specimens were examined under scanning electron microscopy (SEM). Micrograph analysis showed that the bleaching gel caused changes to the enamel surface and that the application of neutral fluoride gel during or after treatment did not completely reverse the changes caused by bleaching. Carbamide peroxide increased enamel porosity. Application of neutral fluoride gel during or after the treatment did not produce any significant decrease in enamel porosity. Total enamel recovery seems to be more strongly associated with the buffering properties of saliva than with the use of different fluoride application protocols.
Keywords: Tooth bleaching, peroxide, fluoride.
RESUMO
Este estudo avaliou o efeito de um gel de peróxido de carbamida a 10% sobre a superfície do esmalte e a capacidade de remineralização da aplicação de flúor neutro durante e após o tratamento de clareamento. Dezesseis fragmentos foram obtidos de quatro dentes humanos extraídos (incisivos e molares) e distribuídos em quatro grupos. O grupo I serviu como controle, e os grupos II, III e IV foram submetidos a 8 horas diárias de clareamento com peróxido de carbamida durante 2 semanas. O grupo III foi tratado com gel de flúor neutro a 2% por 4 minutos diariamente, e o grupo IV recebeu uma aplicação de flúor ao final de 2 semanas de tratamento clareador. Os espécimes foram examinados sob microscopia eletrônica de varredura (MEV). A análise das fotomicrografias mostrou que o gel clareador causou alterações na superfície do esmalte e que a aplicação do gel de flúor neutro durante ou após o tratamento clareador não reverteu completamente as alterações causadas pelo procedimento clareador. O peróxido de carbamida aumentou a porosidade do esmalte. A aplicação de gel de flúor neutro durante ou depois do tratamento não produziu um aumento significativo na porosidade do esmalte. A recuperação total do esmalte parece estar mais associada com a capacidade tampão da saliva do que com o uso de diferentes protocolos de aplicação de flúor.
Palavras-chave: Clareamento dental, peróxido, flúor.
INTRODUCTION
The search for a younger and more pleasant appearance is currently a widespread concern, and many patients seek dental bleaching treatments to obtain an esthetic smile, which is understood as synonymous with health.
Although tooth whitening techniques have been known since the 19th century, tooth bleaching was not used extensively until recently. A review of the literature on the history, safety and effectiveness of current bleaching techniques1 revealed that the first documented use of tooth whitening agents dated back to the mid 1800s and described the use of oxalic acid. Shortly after, in 1884, the first report of the use of peroxide as a bleaching agent was published. In 1895, the use of pyrozone mouth rinsing (25% hydrogen peroxide and ether at a 5:1 ratio) was found to cause bleaching of vital teeth as a side effect. In 1937, the associated use of a heated instrument to speed up the bleaching process was suggested. In 1962, patients who were prescribed the Gly-oxide antiseptic solution (containing 10% carbamide peroxide) for use as a mouth rinse also experienced tooth whitening. In 1989, Haywood and Heymann2 reported on a vital bleaching technique in which patients used a night guard with 10% carbamide peroxide gel. Thereafter, the use of 10% carbamide peroxide has become widespread worldwide as an effective tooth bleaching treatment3.
The mechanism of action of bleaching agents is based on the oxidation of macromolecules responsible for enamel and dentin pigments. The permeability of dental structures provides free access of carbamide peroxide through the enamel and dentin organic matrix4,5. These agents can bleach teeth that are stained with either extrinsic or intrinsic pigments (congenital or traumatic etiology).
The findings of a previous study using scanning electron microscopy (SEM) showed that the use of 10% carbamide peroxide for 5 weeks did not cause changes in enamel texture or surface topography and that the effects of the bleaching process extended to tooth portions that were not in direct contact with the gel2. However, topographic changes, enamel porosity, absence of an enamel prism pattern, changes in calcium, phosphate and potassium levels in the hard tissues and decreased enamel microhardness have been reported after dental exposure to carbamide peroxide bleaching agents1,4,6-10. Other studies show that saliva has a buffering capacity that is responsible for increasing enamel microhardness and that the pH of saliva increases during the treatment11.
Bleaching therapies change the permeability of tooth structures, making them more porous and vulnerable to the penetration of substances, bacteria and stains12-14. Fluoride penetrates the dental tubules and forms insoluble calcium fluoride crystals, which promote desensitization and remineralization of dental tissues14.
When evaluating the remineralizing capacity of different fluoride treatments on carbamide peroxide-bleached enamel, Attin et al.15 found that bleaching caused a significant decrease in enamel hardness and that nonfluoridated bleached enamel presented greater hardness loss when compared with fluoridated bleached enamel. The authors concluded that the remineralization of bleached enamel is improved by the application of highly concentrated fluorides. Fluoride application after bleaching promotes remineralization of the enamel surface via the formation of apatite fluoride, which preserves enamel hardness and resistance to acid attacks. Fluoride also acts on dentin by depositing calcium fluoride, thus reducing the thermal sensitivity caused by bleaching14-18.
The objective of this study was to assess the effect of a 10% carbamide peroxide gel on enamel surface and the remineralizing capacity of neutral fluoride applied either during or after the bleaching treatment.
MATERIAL AND METHODS
Four human teeth (two maxillary central incisors and two mandibular molars), extracted for periodontal reasons were selected for this study. Teeth were stored at 4 ºC in saline solution, which was changed weekly until the experiment began. Written informed consent was obtained from the patients for the donation and use of teeth for research purposes.
After cleaning with water/pumice slurry, the roots were removed with a watercooled diamond disk at low speed. The incisor and molar crowns were bisected in both mesiodistal and incisogingival directions, thus resulting in four fragments per tooth. The 16 fragments were randomly assigned to four groups (n=4) and kept in plastic containers. Bleaching treatment lasted for 2 weeks, with an extra step after the second week for group IV, as follows: Group I, mesioincisal incisor and mesiobuccal molar fragments served as controls and were stored in artificial saliva at 37 ºC, changed daily throughout the experiment; Group II, distoincisal incisor and distobuccal molar fragments were treated with 10% bleaching gel (Whiteness Perfect, FGM, Joinville, SC, Brazil) for 8 hours/ day and stored in artificial saliva at 37 ºC (changed daily) for the remaining 16 hours of each day; Group III, distocervical incisor and distolingual molar fragments were treated with 10% bleaching gel (Whiteness Perfect, FGM, Joinville, SC, Brazil) for 8 hours/day, followed by application of a transparent 2% neutral sodium fluoride gel (SS White, Rio de Janeiro, RJ, Brazil) for 4 minutes, and then stored in artificial saliva at 37 ºC (changed daily) for the rest of the day. Group IV, mesiocervical incisor and mesiolingual molar fragments were treated with 10% bleaching gel (Whiteness Perfect, FGM, Joinville, SC, Brazil) for 8 hours/day and stored in artificial saliva at 37 ºC (changed daily) for the remaining 16 hours. However, in group IV, at the end of the second week of bleaching treatment, fragments were additionally treated with 2% neutral sodium fluoride gel (SS White, Rio de Janeiro, RJ, Brazil) for 4 minutes (one session only).
The bleaching agent was a 10% carbamide peroxide gel, with neutral pH, containing potassium ions, glycol, as well as potassium nitrate and sodium fluoride as desensitizing agents. The bleaching gel was removed from the specimens with a toothbrush under running water. Excess fluoride was eliminated using water-soaked gauze.
Once the treatment protocol was concluded, specimens were examined with a scanning electron microscope (XL20, Philips Electronics NV, Eindhoven, Netherlands) to detect morphological changes on the enamel surface of specimens in the four groups. SEM micrographs were taken at X1000 magnification and analyzed to compare the effect of treatment on enamel in each group.
RESULTS
The analysis of SEM micrographs revealed differences on the enamel surface in the comparison of specimens before bleaching (Figure 1 and Figure 2) and after the 8-hour daily bleaching regimen maintained over a 2-week period, with an increase in enamel surface porosity (Figure 3 and Figure 4).


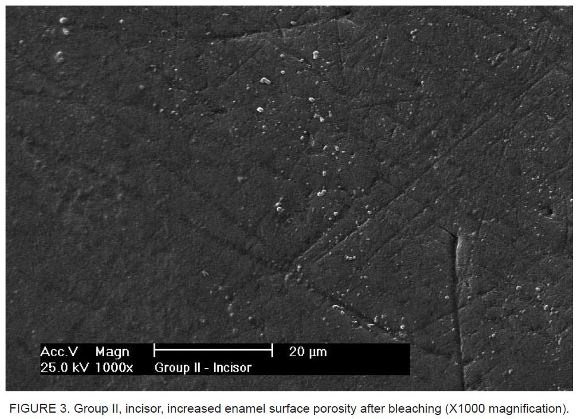
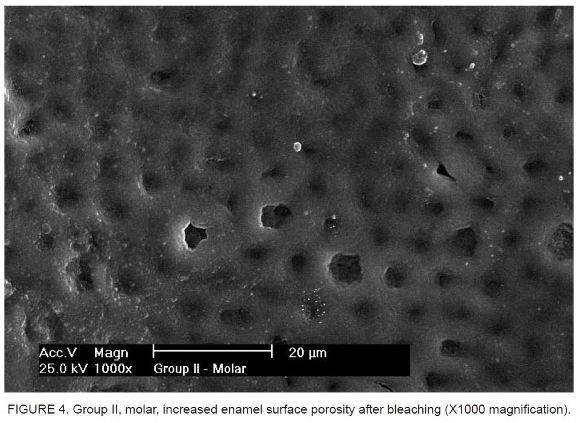
Figure 5 and Figure 6 show enamel surfaces after a 2-week bleaching treatment combined with 4-minute applications of 2% neutral fluoride gel after each bleaching session.

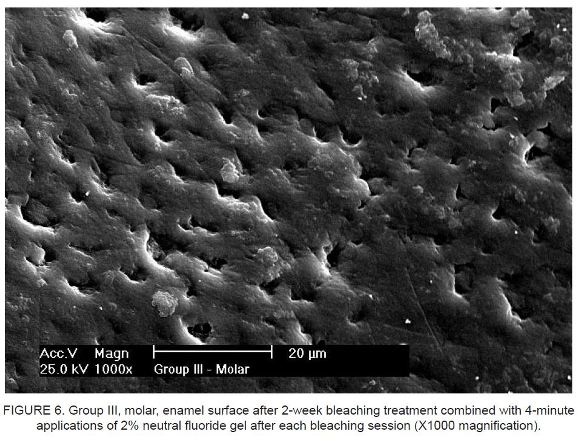
Figure 7 and Figure 8 show enamel surfaces that underwent bleaching and received one single application of fluoride gel at the end of the 2-week bleaching protocol.

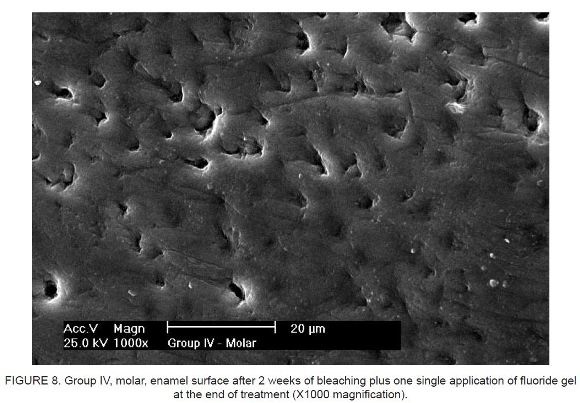
The application of neutral fluoride gel during or after the bleaching treatment did not completely reverse the changes caused by bleaching. No significant differences were observed between the daily application of fluoride gel vs. the application of one single dose of fluoride.
DISCUSSION
Patients are increasingly concerned with having whiter teeth, and esthetic dentistry has been focused on the development of minimally invasive techniques that allow to meet this demand. Among such techniques, perhaps the most popular and widely used one is tooth bleaching, both at home and in the office19.
Ten percent carbamide peroxide has been widely used in at-home bleaching treatments. Haywood and Heymann19 have introduced the technique in which a 0.5- mm-thick guard is used overnight to keep the gel in contact with the teeth for 4 to 6 hours over a 2- to 5-week period.
Evidence shows that 10% carbamide peroxide causes changes on enamel surface and that sodium fluoride is indicated as an adjuvant treatment to minimize the sensitivity caused by carbamide peroxide bleaching. The present study was designed to investigate changes in enamel properties caused by bleaching and whether such changes are affected by the application of neutral sodium fluoride gel either during the treatment or at the end of the bleaching protocol.
Experimental studies have described changes in the texture of bleached enamel. Although Haywood et al.2 have found no changes in enamel texture or topography after treatment with 10% carbamide peroxide, McGucking et al.20 and Groebler et al.10 measured the pH of several commercial brands of tooth bleaching agents and observed that all products produced enamel porosities, in spite of a neutral or nonneutral pH. Other studies12-14,21 have also reported changes the enamel surface, such as increased porosity and altered prism structure. Finally, previous SEM studies7,22 have shown significant surface changes in enamel topography after a 4-week bleaching protocol, especially when low pH bleaching gels were used.
The comparison between specimens that received and those that did not receive fluoride treatment in our sample revealed that fluoride application did not promote significant enamel recovery. Despite a decrease in pore size, the porosity pattern remained in all groups. Attin et al.15, Martin et al.22 and Borges et al.18 have tested different types of fluoride for the remineralization of bleached enamel. All those authors concluded that the application of high concentrations of fluoride significantly increased enamel hardness, and recommended the application of fluoride after exposure to bleaching agents to promote enamel remineralization. However, the findings of our study suggest that the remineralizing effect of topical fluoride on the bleached enamel is neutralized by a new application of the bleaching gel. Similarly, a single application of fluoride at the end of treatment failed to provide any significant gain in terms of bleached enamel recovery.
Finally, the comparison between incisor and molar fragments showed that the enamel from these teeth responded differently to the bleaching treatment. SEM micrographs revealed that porosities were more evident on the enamel of molar teeth, suggesting that this tooth may be more porous originally. These results are in agreement with a previous investigation7 that also used incisors and molars and reported a similar appearance for bleached enamel as observed in our study.
CONCLUSIONS
The SEM examination of 10% carbamide peroxide-bleached enamel surfaces revealed that this bleaching agent increased enamel porosity, as expected. Moreover, the daily application of neutral fluoride gel during the treatment did not produce significant reductions in enamel porosity, and the use of neutral fluoride gel at the end of the bleaching protocol was not able to completely reverse the changes on enamel surface.
Our findings seem to suggest that total enamel recovery may occur regardless of fluoride application, and that it is more likely to be associated with the buffering properties of saliva. Further studies should be conducted with different materials and compositions to corroborate or confront these outcomes.
REFERENCES
1. Oliveira R, Paes Leme AF, Giannini M. Effect of a carbamide peroxide bleaching gel containing calcium or fluoride on human enamel surface microhardness. Braz Dent J. 2005;16(2):103-6. [ Links ]
2. Haywood VB, Leech T, Heymann HO, Crumpler D, Bruggers K. Nightguard vital bleaching: effects on enamel surface texture and diffusion. Quintessence Int. 1990;21(10):801-4. [ Links ]
3. Haywood VB. History, safety, and effectiveness of current bleaching techniques and applications of the nightguard vital bleaching technique. Quintessence Int. 1992;23(7):471-88. [ Links ]
4. Josey AL, Meyers IA, Romaniuk K, Symons AL. The effect of a vital bleaching technique on enamel surface morphology and the bonding of composite resin to enamel. J Oral Rehabil. 1996;23(4):244-50. [ Links ]
5. Seghi RR, Denry I. Effects of external bleaching on indentation and abrasion characteristics of human enamel in vitro. J Dent Res. 1992;71(6):1340-4. [ Links ]
6. Rotstein I, Dankner E, Goldman A, Heling I, Stabholz A, Zalkind M. Histochemical analysis of dental hard tissues following bleaching. J Endod. 1996;22(1):23-5. [ Links ]
7. Shannon H, Spencer P, Gross K, Tira D. Characterization of enamel exposed to 10% carbamide peroxide bleaching agents. Quintessence Int. 1993;24(1):39-44. [ Links ]
8. Smidt A, Weller D, Roman I, Gedalia I. Effect of bleaching agents on microhardness and surface morphology of tooth enamel. Am J Dent. 1998;11:83-5. [ Links ]
9. Fu B, Hoth-Hannig W, Hannig M. Effects of dental bleaching on micro and nanoorphological alterations of the enamel surface. Am J Dent. 2007;20(1):35-40. [ Links ]
10. Grobler SR, Majeed A, Moola MH. Effect of various tooth-whitening products on enamel microhardness. SADJ. 2009;64(10):474-9. [ Links ]
11. Leonard RH, Bentley CD, Haywood VB. Salivary pH changes during 10% carbamide peroxide bleaching. Quintessence Int. 1994;25(8):547-50. [ Links ]
12. Ben-Amar A, Liberman R, Gorfi l C, Bernstein Y. Effect of mouthguard bleaching on enamel surface. Am J Dent. 1995;8(1):29-32. [ Links ]
13. Bitter NC, Sanders JL. The effect of bleaching agents on the enamel surface: a scanning electron microscopic study. Quintessence Int. 1993;24:817-24. [ Links ]
14. Trowbridge H, Silver D. A review of current approaches to in-office management of tooth hypersensitivity. Dent Clin North Am 1990;34(3):561-81. [ Links ]
15. Attin T, Kielbassa AM, Schwanenberg M, Hellwig E. Effect of fluoride treatment on remineralization of bleached enamel. J Oral Rehabil. 1997;24(4):282-6. [ Links ]
16. Haywood VB, Parker MH. Nightguard vital bleaching beneath existing porcelain veneers: a case report. Quintessence Int. 1999;30(11):743-7. [ Links ]
17. Chen HP, Chang CH, Liu JK, Chuang SF, Yang JY. Effect of fluoride containing bleaching agents on enamel surface properties. J Dent. 2008;36(9):718-25. [ Links ]
18. Borges AB, Yui KC, D’Avila TC, Takahashi CL, Torres CR, Borges AL. Influence of remineralizing gels on bleached enamel microhardness in different time intervals. Oper Dent. 2010;35(2):180-6.
19. Haywood VB, Heymann HO. Nightguard vital bleaching. Quintessence Int. 1989;20(3):173-6. [ Links ]
20. McGuckin RS, Babin JF, Meyer BJ. Alterations in human enamel surface morphology following vital bleaching. J Prosthet Dent. 1992;68(5):754-60. [ Links ]
21. Sasaki RT, Arcanjo AJ, Flório FM, Basting RT. Micromorphology and microhardness of enamel after treatment with home-use bleaching agents containing 10% carbamide peroxide and 7.5% hydrogen peroxide. J Appl Oral Sci. 2009;17(6):611-6. [ Links ]
22. Martin JM, Almeida JB, Rosa EA, Soares P, Torno V, Rached RN, et al. Effect of fluoride therapies on the surface roughness of human enamel exposed to bleaching agents. Quintessence Int. 2010;41(1):71-8. [ Links ]
 Corresponding Author:
Corresponding Author:
Roberta de Azambuja Tagliari
Universidade Luterana do Brasil
Rua Benjamin Constant 454/801, bairro Centro
CEP 99010–130 – Porto Alegre, RS – Brazil
Tel./fax: +55 (54) 3313.3925
E-mail:rtagliari@gmail.com













