Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Stomatos
versão impressa ISSN 1519-4442
Stomatos vol.18 no.34 Canoas Jan./Jun. 2012
SCIENTIFIC ARTICLE
Immunohistochemical Analysis of Metalloproteases in Dentigerous Cysts, Radicular Cysts and Keratocystic Odontogenic Tumors: Systematic Review
Análise Imunoistoquímica de Metaloproteases nos Cistos Dentígeros, Cistos Radiculares e Tumores Odontogênicos Ceratocísticos: Revisão Sistemática
Rafaela Scariot1; Imara Castro Morosini1; Cassius Carvalho Torres-Pereira2; José Miguel Céspedes Amenabar2; Nelson Luis Barbosa Rebellato3; Renato Cordeiro Gugisch4
1DDS, Students of Master’s Program in Dentistry, Universidade Federal do Paraná, Curitiba, Brazil.
2Professors, Oral Diagnoses, Department of Stomatology, School of Dentistry, Universidade Federal do Paraná, Curitiba, Brazil.
3Professor, Oral and Maxillofacial Surgery and Trauma, Department of Stomatology, School of Dentistry, Universidade Federal do Paraná, Curitiba, Brazil.
4Professor, Odontopediatrics, Department of Stomatology, School of Dentistry, Universidade Federal do Paraná, Curitiba, Brazil.
ABSTRACT
Evidence suggests that metalloprotease expression may affect the biological behavior of odontogenic lesions. This study was conducted to review the literature about the role of metalloproteases in the development of odontogenic lesions. A search was carried out using one database, MEDLINE, via PubMed. Only articles written in English were included. Abstracts of all articles retrieved in the electronic search were evaluated for their relevance. Three articles met inclusion criteria. They analyzed the role of MMP-2, MMP-8 and MMP-13 in radicular cysts, dentigerous cysts and keratocystic odontogenic tumors, and of MMP-1, MMP-7 and MMP-27 in keratocystic odontogenic tumors. The immunostaining technique used for all studies was similar, differing only in type of staining used. Different immunoreactivity results were found in the studies. The pattern of metalloprotease expression in odontogenic lesions was different from the pattern found in other lesions. In the studies analyzed, there was a significant positive immunoreactivity for metalloproteases in odontogenic lesions, particularly in keratocystic odontogenic tumors, a finding that may explain KCOT aggressiveness.
Keywords: matrix metalloproteases, extracellular matrix, odontogenic lesions.
RESUMO
Evidências sugerem que a expressão das metaloproteinases podem afetar o comportamento biológico das lesões odontogênicas. Esse estudo foi conduzido a fim de revisar a literatura sobre o papel das metaloproteinases no desenvolvimento das lesões odontogênicas. A pesquisa foi realizada utilizando a base de dados do MEDLINE, via PUBMED. Somente artigos escritos em língua inglesa foram aceitos. Os resumos de todos os artigos encontrados na busca foram avaliados de acordo com sua relevância. Três artigos preencheram os critérios de inclusão. Eles analisaram o papel da MMP-2, MMP-8 e MMP-13 nos cistos radiculares, cistos dentígeros e nos tumores odontogênicos ceratocísticos (TOC) e MMP-1, MMP-7 e MMP-27 no TOC. A técnica imunoistoquímica utilizada por todos os estudos foi similar, diferindo somente pelo tipo da coloração utilizada. Diferentes imunomarcações foram encontradas nos estudos. O padrão da expressão das metaloproteinases nas lesões odontogênicos variou entre as lesões. Nos estudos analisados, houve uma imunomarcação positiva, significante estatiticamente, das metaloproteinases nas lesões odontogênicas em especial nos TOCs, o que pode explicar a agressividade dessas lesões.
Palavras-chave: metaloproteinases da matriz, matriz extracelular, lesões odontogênicas.
INTRODUCTION
OThe molecules of the extracellular matrix play an important role during cell development and morphogenesis. Matrix metalloproteases (MMPs) are zinc-dependent proteinases that participate in extracellular matrix degradation1-3 and favor the invasion and proliferation of tumor cells4. Under normal physiological condition, MMPs are weakly expressed in tissues, whereas in pathological events, their overexpression is the cause of the imbalance between MMPs and tissue inhibitors of MMPs (TIMPs)5-6-7.
To this date, 28 genes of the metalloprotease family have been identified in human beings. They are classified into 5 groups according to substrate specificity and internal homology: collagenases, gelatinases, stromelysins, membrane-type MMPs, and others, including matrilysins8.
Some studies about odontogenic cysts and tumors have been conducted to evaluate metalloprotease expression in these lesions. MMP-1, MMP-2, MMP-3, MMP-8, MMP-9 and MMP-13 have been identified in odontogenic cysts, which suggests that MMPs play an important role in the growth of these tumors9-10.
MMP-2 and MMP-9 are gelatinases of the MMP family that degrade some types of collagen, such as type IV, V and X collagen, and denature type I, II and III fibrillar collagen. They have been found on the walls of odontogenic cysts and in cystic fluid. In addition, they seem to be involved in the pathologic process of cyst expansion. Both MMP-2 and MMP-9 are secreted in their inactive form and have to be converted into their active form to perform their function. Studies found that the MMP-9 activity rate is directly associated with the type of odontogenic cyst9-11.
MMP- and MMP-13, also known as collagenases, may directly initiate a critical breakdown of collagen, both in benign and malignant bone-destructive lesions12.
Other studies also correlate matrilysins (MMP-7 and MMP-26) with lesions of odontogenic origin, particularly keratocystic odontogenic tumors (KCOT)13. Matrilysins seem to degrade the substrate of the basement membrane and are correlated with cell proliferation, apoptosis, invasion and metastases.
Because of the importance of MMPs in the regulation, integrity and composition of the extracellular matrix and the possible correlation of MMPs with pathological processes, this study reviewed findings in the literature about this topic to improve our understanding about the biological processes involved in the development of odontogenic lesions.
METHODS
Review strategy
The studies were selected using the MEDLINE database (via PubMed). Only studies published between 1990 and 2009 were included. The following keywords were used: (metalloproteinase “OR” metalloproteinases “OR” metalloprotease “OR” metalloproteases “OR” MMP “OR” MMPs) AND odontogenic AND (cyst “OR” cysts “OR” keratocyst “OR” keratocysts). After the list of references was retrieved, only studies published in English were included in the review.
Criteria for sample selection
The title and abstract of all studies retrieved in the electronic search were evaluated according to their adequacy. The full texts of the studies selected were reviewed, and a decision was made about their eligibility for inclusion. To be eligible for data extraction, the studies had to meet the following criteria: 1) original research studies; 2) studies with immunohistochemical analyses; 3) inclusion of at least one type of metalloprotease in the study; and 4) inclusion of at least one of the three diseases under analysis (radicular cyst, dentigerous cyst, KCOT).
Data extraction
Of the studies selected, the following main data were extracted: 1) Country of origin; 2) Metalloproteases included; 3) Sample composition and size; 4) Methods used; and 5) Results found.
RESULTS
The MEDLINE search (via PubMed) yielded 15 potentially eligible studies. Their abstracts were selected according to their relevance. After screening, 11 studies remained for full text analysis. The four studies excluded in the first analysis14-15-16-17 dealt with metalloprotease expression in lesions not evaluated in this study. After reading the full texts, 8 studies were classified as inadequate and excluded: 0511-18-19-20-21 had no immunohistochemical analyses; 0222-23 did not involve metalloproteases directly; and 0124 was a literature review. Therefore, three studies met inclusion criteria and were analyzed for data extraction (Graph 1).
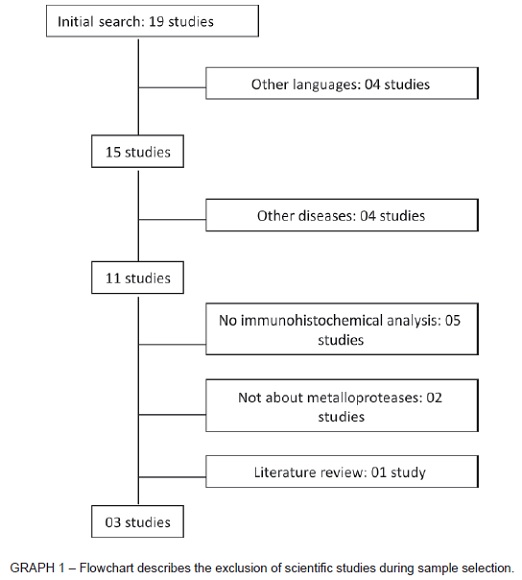
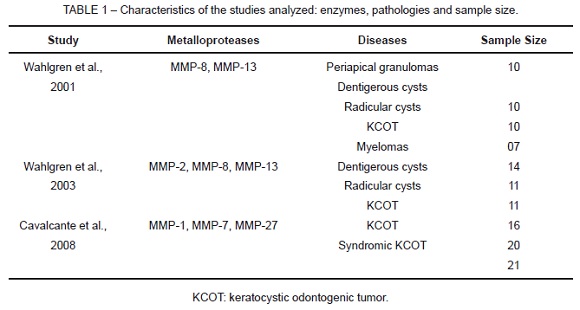
Of the studies analyzed, two were conducted in Finland and one, in Brazil. Mean number of authors per study was 08. The metalloproteases, the lesion evaluated in each study and the sample size are shown in Table 1. MMP-2, MMP-8 and MMP-13 were analyzed in radicular cysts, dentigerous cysts and KCOT. In addition, MMP-1, MMP-7 and MMP-27 were analyzed in KCOTs.
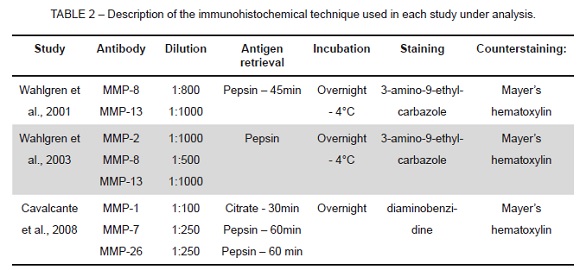
The methods used for the immunohistochemical analysis in each study are shown in Table 2, which describes antibody, dilution, antigen retrieval, incubation, staining and counterstaining. The method used in all studies was similar and only staining was different.
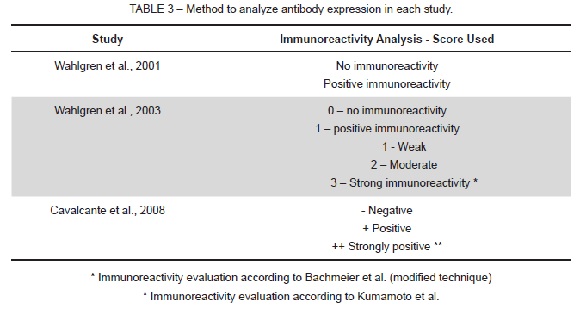
The methods to analyze the antibodies expressed in each study are shown in Table 3. Three different methods were used to evaluate immunoexpression, and accuracy increased with publishing date.
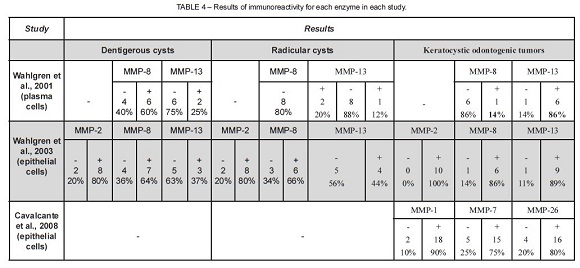
Because of the difficulty in standardizing the analysis of immunoexpression, results were classified as: no immunoreactivity (-) or positive immunoreactivity (+). When immunoreactivity was present, its intensity was not analyzed. Results of immunoreactivity for each enzyme in the studies are summarized in Table 4. The pattern of metalloprotease expression in KCOTs is different from that found in other lesions.
DISCUSSION
The scarcity of studies that investigate the association between metalloproteases and lesions of odontogenic origin explains the small number of studies retrieved in this review. Although the search was applied in different ways, there were no changes in results. In general, very few studies have been conducted to evaluate the presence of these enzymes in other lesions, which may be explained by the fact that the discovery of these enzymes is recent. MMP-1 was first described by Jerome Gross and Charles Lapiere in 1962. They found enzymatic activity (collagen triple-helix degradation) during the metamorphosis of a tadpole tail25. This enzyme was called interstitial collagenase. Currently, the descriptions of 28 different types of metalloproteases are found in the literature, and MMP-28 was only discovered in 200126.
After the initial selection of studies (n=15), only 03 met the criteria for data extraction. Coincidently, two studies had been conducted by the same research team. In the final selection, the immunohistochemical evaluation of different cell types, plasma cells and epithelial cells, were also analyzed. Initially, the type of cell under analysis would be an exclusion criterion, and only the analysis of epithelial cells would be included. However, the inclusion of both types was possible because of the small number of studies about the topic of interest.
Several metalloproteases were evaluated in the studies selected. These enzymes are subdivided into six groups according to their substrate and to the similarity of their structural domain. The groups analyzed in this study were collagenases (MMP-1, MMP-8 and MMP-13), gelatinases (MMP-2) and matrilysins (MMP-7 and MMP-26). Collagenases are enzymes that can break the links that make up type I, II and II interstitial collagen, as well as other components of the extracellular matrix. Gelatinases can digest collagen after degradation3. MMP-2 is directly associated with osteogenesis27. Matrilysins are proteases that contribute to the degradation of the cell membrane and are involved in several processes, such as cell proliferation, apoptosis, invasion and metastases13. All these enzymes are directly associated with physiological processes, but are overexpressed in pathological events.
Radicular cysts are classified as inflammatory cysts that originate from the epithelial cell rests of Malassez, secondary to pulp necrosis28. Dentigerous cysts are developmental cysts, usually asymptomatic, that may potentially lead to cortical expansion and bone fenestration. They are associated with the crown of an impacted tooth. The exact pathogenesis of dentigerous cysts remains unknown, but several authors believe that they develop from tooth follicles29. Some studies evaluated epithelial and mesenchymal characteristics of different cysts to elucidate questions that remain unclear in the literature. Some proteins and enzymes of the extracellular matrix seem to be associated with the development and biological behavior of these cysts. The variable patterns of metalloprotease expression in cyst epithelium indicate that they have different roles in regulating proliferation, maturation and cell migration. In the studies reviewed, immunoreactivity of dentigerous and radicular cysts for MMP-2, MMP-8 and MMP-13 was found in two different studies. MMP-2 (gelatinase) expression in dentigerous cysts reached 80% immunoreactivity, whereas for MMP-8 and MMP-13, it was about 60% and 30%. The low immunoexpression of MMP-13, in both dentigerous and radicular cysts, may be associated with its role in bone-destructive lesions. MMP-13 is associated with the uncontrolled destruction of the extracellular matrix and of the bilaminar zone in aggressive malignant lesions12.
KCOT is a benign tumor with high recurrence rates. According to several studies, recurrence ranges from 3% to 60%30. Keratocysts were reclassified as keratocystic odontogenic tumors by the World Health Organization (WHO) due to their aggressive behavior and histology and new information about their genetics31-32-33. The WHO defi nes them as benign uni- or multicystic tumors of odontogenic origin with a characteristic lining of parakeratinized stratified squamous epithelium and a potential for aggressive, infiltrative behavior.
New information about KCOT, particularly regarding bone resorption and growth, have led to changes in its treatment. Several recent studies focused on the collagenolytic activity of this tumor because this same activity is not found in other odontogenic lesions. Also, the activity of collagenase in tissues is strongly controlled by a complex regulating system that may have collagenolytic effects and, therefore, influence the expansion of the bone caused by the cyst24 Some studies evaluated the presence, activity, activation and inhibition of extracellular matrix metalloproteases in KCOT to define the role of these enzymes in the molecular mechanisms associated with cystic growth. The study under analysis here also evaluated MMP-1, MMP-2, MMP-8, MMP-13 and MMP-27 in KCOTs. Strong immunoreactivity for these enzymes was found in all studies, except for MMP-8 in only one study. This may be explained by the fact that the analysis was conducted using plasma cells, and not epithelial cells. Differentiated plasma cells produce cytokines, such as interleukin-1, found in cystic fluid and responsible for MMP-9 and MMP-13 regulation10. MMP-8 has a role in focal remodeling and cystic epithelial growth, but does not participate in the modulation of molecules in the bilaminar zone12.
In the studies under analysis, there was a stronger expression of MMP-2 and MMP-13 in KCOTs than in dentigerous and radicular cysts. MMP-2 and MMP-13 are produced by the cystic epithelium and are found in the bilaminar zone. Some authors10 suggest that these enzymes may induce epithelial migration due to the fragmentation of laminin-5 (transmembrane protein found exclusively in the bilaminar zone) and that this may explain the stimulation of migration and the growth potential of KCOTs. They also found that MMP-2 may activate latent MMP-13, which suggests that this enzyme may act as a cascade in the activation of epithelial migration, induced by fragments of degraded laminin. They concluded that the fast proliferation of the epithelium and the tendency to separate from the connective tissue capsule may be associated with laminin-5 modulation by MMP-2 and MMP-13 in KCOTs.
Immunoreactivity for MMP-1 was found in 90% of the cases, and it was the second strongest immunoreactivity in all studies under analysis. Type I collagen, responsible for the strength and rigidity of the connective tissue, is the main organic matrix of bone34, and MMP-1 is one of the proteases that may degrade the triple-helix domain of type I collagen35-36. The presence of MMP-1 in KCOTs may be associated with the organic degradation of the bone matrix, which favors the dissemination of the disease through the trabecular spaces13. According to some authors, it is still too early to suggest that a patient with strong MMP-1 immunoexpression should be warned about possible greater susceptibility to the development of the nevoid basal cell carcinoma syndrome13. However, future studies may use immunoreactivity for some enzymes to clarify diagnoses and plan treatments.
Positivity was found for MMP-7 and MMP-27, the matrilysins, in 75% and 80% of the cases. These enzymes are associated with basement membrane degradation. In addition, they can activate other metalloproteases, such as MMP-2 and MMP-9 (gelatinases). These gelatinases degrade type IV collagen and basement membrane laminin13. The role of matrilysins in odontogenic lesions has been evaluated only by the same research team, which precludes further scientifi c correlations.
CONCLUSIONS
• Further studies should be conducted to evaluate the correlations between metalloproteases and odontogenic lesions.
• In the studies analyzed, an important immunoreactivity for metalloproteases was found in odontogenic lesions, particularly in KCOTs.
• KCOTs have a more aggressive biological behavior, which may be currently explained by immunoreactivity for several proteins and enzymes, particularly
metalloproteases.
REFERENCES
1. Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004,16:558-64. [ Links ]
2. Nagase H, Woessner JF. Matrix metalloproteinases. J Bio Chem. 1999,274(214):491-4. [ Links ]
3. Visse R, Nagase H. Matrix metalloprooteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003,92:827-39. [ Links ]
4. Souza AP, Line SRP. The biology of matrix metalloproteinases. Rev FOB. 2002,10:1-6. [ Links ]
5. Pereira ALA, Veras SLS, Silveira EJD, Seabra FRG, Pinto LP, Souza LB, Freitas RA. O papel das proteínas da matriz extracelular e das metaloproteinases em carcinomas de cabeça e pescoço: uma atualização bibliográfica. Rev Bras Otorrinolaringol. 2005,71:81-6. [ Links ]
6. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2004, 69:222-31. [ Links ]
7. Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bio-org Med Chem. 2007, 15:2223-68. [ Links ]
8. Navarro VP, Nelson-Filho P, Silva LAB, Freitas AC. A participação das metaloproteinases da matriz nos processos fisiopatológicos da cavidade bucal. Rev Odont UNESP. 2006, 35(4):233-8. [ Links ]
9. Teronen O, Salo T, Konttinen YT, Rifkin B, Vernillo A, Ramamurthy NS, Kjeldsen L, Borregaard N, Hietanen J, Sorsa T. Identification and characterization of gelatinases tipo IV collagenases in jaw cyst wall. J Oral Pathol Med. 1996, 24:78-84. [ Links ]
10. Wahlgren J, Väänänen A, Teronen O, Sorsa T, Pirilä E, Hietanen J, Maisi P, Tjäderhane L, Salo T. Laminin-5 gamma 2 chain is colocalized with gelatinase-A (MMP-2) and collagenase-3 (MMP-13) in odontogenic keratocysts. J Oral Pathol. 2003, 32(2):100-7. [ Links ]
11. Kubota Y, Ninomiya T, Oka S, Takenoshita Y, Shirasuna K. Interleukin-1 alphadependent regulation of matrix metalloproteinase-9 (MMP-9) secretion and activation in the epithelial cells of odontogenic jaw cysts. J Dent Res. 2000, 79(6): 1423-1430. [ Links ]
12. Wahlgren J, Maisi P, Sorsa T, Sutinen M, Tervahartiala T, Pirilä E, Teronen O, Hietanen J, Tjäderhane L, Salo T. Expression and induction of collagenases (MMP-8 and -13) in plasma cells associated with bone-destructive lesions. J Pathol. 2001. 194(2):217-24. [ Links ]
13. Cavalcante RB, Pereira KM, Nonaka CF, Nogueira RL, de Souza LB. Immunohistochemical expression of MMPs 1, 7, and 26 in syndrome and nonsyndrome odontogenic keratocysts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008, 106(1) 99-105. [ Links ]
14. Gong Y, Wang L, Wang H, Li T, Chen X. The expression of NF-kappaB, Ki-67 and MMP-9 in CCOT, DGCT and GCOC. Oral Oncol. 2008, 45(6):515-20. [ Links ]
15. Sekine S, Takata T, Shibata T, Mori M, Morishita Y, Noguchi M, Uchida T, Kanai Y, Hirohashi S. Expression of enamel proteins and LEF1 in adamantinomatous craniopharyngioma: evidence for its odontogenic epithelial differentiation. Histopathology. 2004, 45(6):573-9. [ Links ]
16. Takata T, Zhao M, Uchida T, Wang T, Aoki T, Bartlett JD, Nikai H. Immunohistochemical detection and distribution of enamelysin (MMP-20) in human odontogenic tumors. J Dent Res. 2008, 79(8):1608-13. [ Links ]
17. Takata T, Zhao M, Nikai H, Uchida T, Wang T. Ghost cells in calcifying odontogenic cyst express enamel-related proteins. Histochem J. 2000, 32(4):223-9. [ Links ]
18. Kubota Y, Nitta S, Oka S, Nakagawa S, Ninomiya T, Shirasuna K. Discrimination of ameloblastomas from odontogenic keratocysts by cytokine levels and gelatinase species of the intracystic fluids. J Oral Pathol Med. 2001, 30(7): 421-7. [ Links ]
19. Teronen O, Salo T, Laitinen J, Törnwall J, Ylipaavalniemi P, Konttinen YT, Hietanen J, Sorsa T. Characterization of interstitial collagenases in jaw cyst wall. Eur J Oral Sci. 1995, 103(3):141-7. [ Links ]
20. Kubota Y, Oka S, Nakagawa S, Shirasuna K. Interleukin-1alpha enhances type I collagen-induced activation of matrix metalloproteinase-2 in odontogenic keratocyst fibroblasts. J Dent Res. 2002, 81(1):23-7. [ Links ]
21. Oka S, Kubota Y, Yamashiro T, Ogata S, Ninomiya T, Ito S, Shirasuna K. Effects of positive pressure in odontogenic keratocysts. J Dent Res. 2005, 84(10):913-8. [ Links ]
22. Ali, MAA. Expression of extracellular matrix metalloproteinase inducer in odontogenic cysts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008, 106(2):258-63. [ Links ]
23. Meghji S, Henderson B, Bando Y, Harris M. Interleukin-1: the principal osteolytic cytokine produced by keratocysts. Arch Oral Biol. 1992, 37(11):935-43. [ Links ]
24. Shear M. The aggressive nature of the odontogenic keratocyst: is it a benign cystic neoplasm? Part 1. Clinical and early experimental evidence of aggressive behavior. Oral Oncol. 2002, 38(3):219-26. [ Links ]
25. Gross J, Lapiere C. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc natl Acad Sci USA. 1962, 48:1014-22. [ Links ]
26. Lohi J, Wilson CL, Roby JD, Parks WC. Epilysin, a novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to
injury. J Biol Chem. 2001, 276(13):10134-44.
27. Martignetti JA, Aqeel AA, Sewairi WA, Boumah CE, Kambouris M, Mayouf SA, Sheth KV, Eid WA, Dowling O, Harris J, Glucksman MJ, Bahabri S, Meyer BF, Desnick
RJ. Mutation of the matrix metalloproteinase 2 gene (MMP-2) causes a multicentric osteolysis and arthritis syndrome. Nat Genet. 2001, 28:261-5.
28. Wood RE, Nortjé CJ, Padayachee A, Grotepass F. Radicular cysts of primary teeth mimicking premolar dentigerous cysts: report of three cases. ASDC J Dent Child. 1988, 55:288-90. [ Links ]
29. Koseoglu BG, Atalay B, Erdem MA. Odontogenic cysts: a clinical study of 90 cases. J Oral Sci. 2004, 46:253-7. [ Links ]
30. Shear M. Cysts of the oral regions. 3rd ed. Oxford: Wright, Butterworth-Heinemann, 1992. [ Links ]
31. Jackson IT, Potparic Z, Fasching M, Schievink WI. Penetration of the skull base by dissecting keratocyst. J Cranio-Maxillofac Surg. 1993, 21:319-25. [ Links ]
32. Toller PA. Origin and growth of cysts of the jaws. Ann Roy Coll Surg Eng. 1967, 40:306-36. [ Links ]
33. Madras J, Lapointe H. Keratocystic odontogenic tumour: reclassification of the odontogenic keratocyst from cyst to tumour. J Can Dent Assoc. 2008, 74(2):165–165h.
34. Delaissé JM, Engsig MT, Everts V, Ovejero MC, Ferreras M, Lund L, Vu TH, Werb Z, Winding B, Lochter A, Karsdal MA, Troen T, Kirkegaard T, Lenhard T, Heegaard AM, Neff L, Baron R, Foged NT. Proteinases in bone resorption:obvious and less obvious roles. Clin Chim Acta. 2000, 291:223-34. [ Links ]
35. Kumamoto H, Yamauchi K, Yoshida M, Ooya K. Immunohistochemical detection of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in ameloblastomas. J Oral Pathol Med. 2003, 32:114-20. [ Links ]
36. Pardo A, Selman M. MMP-1: the elder of the family. Int J Biochem Cell Biol. 2003, 37:283-8. [ Links ]
 Corresponding Author:
Corresponding Author:
Rafaela Scariot de Moraes
Rua Dr. Brasílio Vicente de Castro, 320. Apto 403 – Campo Comprido
CEP 81200–526 – Curitiba/PR , Brazil
E-mail: rafaela_scariot@yahoo.com.br













