Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Stomatos
versão impressa ISSN 1519-4442
Stomatos vol.19 no.37 Canoas Jul./Dez. 2013
Antibacterial effect of oral antiseptics on facultative bacteria
Efeito antibacteriano de antissépticos bucais sobre bactérias facultativas
Marcel da Silva Garrote I; Ana Helena Gonçalves de Alencar II; Cyntia Rodrigues de Araújo Estrela III; Hugo Alexandre de Souza II; Denise Ramos Silveira Alves I; Carlos Estrela II
I MSc from Department of Stomatology, Universidade Federal de Goiás (UFGO), Goiânia, GO, Brazil
II PhD from the Department of Stomatology, UFGO, Goiânia, GO, Brazil
III PhD from the Department of Endodontics, Universidade de Cuiabá (UNIC), Cuiabá, MT, Brazil
ABSTRACT
Purpose: To evaluate the antibacterial effect of four oral antiseptics (two solutions of cetylpyridinium chloride, chlorhexidine gluconate and benzalkonium chloride) on facultative bacteria using two methods. Methods: Strains were inoculated in 7 mL of brain heart infusion (BHI) and incubated at 37°C for 24 hours. For the agar diffusion test, 15 Petri plates with 20 mL of brain heart infusion agar (BHIA) were inoculated with 0.1 mL of microbial suspensions using sterile swabs to produce confluent growth; one Petri plate was not inoculated. Thirty-six 9-mm paper discs were immersed in the experimental solutions (0.07% cetylpyridinium chloride, 0.075% cetylpyridinium chloride, 0.12% chlorhexidine gluconate, and 0.13% benzalkonium chloride) for 1 minute. Subsequently, three paper discs containing irrigant solutions were placed on the BHIA in each plate. The plates were kept at room temperature for 1 hour and incubated at 37°C for 48 hours. Two measurements of the inhibition zones were made on the paper discs containing the solutions, and mean values were calculated. For the direct exposure test, 216 #50 sterilized paper points were immersed in the microorganism suspensions for 5 minutes, placed onto Petri plates and covered with 10 mL of irrigant solution. At one, five, 10 and 30 minutes, three paper points were removed from the contact substances, transported individually, immersed in 7 mL Letheen broth and incubated at 37°C for 48 hours. Bacterial growth was evaluated by turbidity. An inoculum of 0.1 mL Letheen broth was transferred to 7 mL BHI, and incubated as described above. Bacterial growth was evaluated according to turbidity. Results: Inhibition zones were greater than 10 mm for all substances and all microorganisms under study. The antibacterial effect of 0.13% benzalkonium chloride against the biological indicators was observed after five minutes in direct exposure, while cetylpyridinium chloride and chlorhexidine gluconate had an antibacterial effect against all the microorganisms after 10 minutes. Conclusion: The antiseptic solutions included in this study had an antibacterial effect against S. mutans, E. faecalis, and P. aeruginosa.
Keywords: Cetylpyridinium chloride, Chlorhexidine, Benzalkonium.
RESUMO
Objetivo:Avaliar o efeito antibacteriano de antissépticos bucais sobre bactérias facultativas por meio de testes de difusão em ágar e teste por exposição direta. Metodologia: Cepas de S. mutans (ATCC 25175), E. faecalis (ATCC 29212) e P. aeruginosa (ATCC 27853) foram inoculadas em 7 mL de brain heart infusion (BHI) e incubadas a 37°C por 24 horas. Para o teste de difusão em ágar, 15 placas de Petri com 20 mL de brain heart infusion agar (BHIA) foram inoculadas com 0,1 mL das suspensões microbianas, com auxílio de swabs esterilizados, de modo a se obter um crescimento confluente e uma placa de Petri não foi inoculada. Trinta e seis discos de papel com 9 mm de diâmetro foram imersos nas soluções experimentais de cloreto de cetilpiridínio 0,07%, cloreto de cetilpiridínio 0,075%, gluconato de clorexidina 0,12% e cloreto de benzalcônio 0,13% durante 1 minuto. A seguir, três discos de papel contendo uma das soluções irrigantes foram colocados sobre a superfície do BHIA. As placas foram mantidas por 1 hora em temperatura ambiente e incubadas a 37°C por 48 horas. Os diâmetros dos halos de inibição microbiana foram medidos valendo-se de duas medidas de forma perpendicular entre si, sendo obtida a média de seus comprimentos. Para o teste de exposição direta, 216 cones de papel absorventes esterilizados nº 50 foram imersos na suspensão de micro-organismos por 5 minutos, e a seguir foram colocados em placas de Petri e cobertos com 10 mL de uma das soluções testes. Em intervalos de 1, 5, 10 e 30 minutos, 3 cones de papel absorventes foram retirados do contato com as substâncias, transportados individualmente e imersos em 7 mL de Letheen Broth, e incubados a 37°C por 48 horas. O crescimento microbiano foi avaliado pela turbidade do meio de cultura. Um inóculo de 0,1 mL obtido do Letheen Broth foi transferido para 7 mL de BHI, e incubado nas mesmas condições descritas. O crescimento microbiano foi novamente avaliado pela turbidade do meio de cultura. Resultados: Os halos de inibição foram maiores que 10 mm para todas as substâncias e em todos os micro-organismos. A solução de cloreto de benzalcônio apresentou efeito antibacteriano contra os indicadores biológicos após 5 minutos em teste por exposição direta, enquanto que o cloreto de cetilpiridínio e o gluconato de clorexidina apresentaram efeito antibacteriano somente após 10 minutos. Conclusão: As soluções antissépticas estudadas apresentaram efeito antibacteriano por contato direto frente S. mutans, E. faecalis e P. aeruginosa.
Palavras-chave: Cloreto de cetilpiridínio, clorexidina, benzalcônio.
INTRODUCTION
The microorganisms in the oral cavity are usually organized in a biofilm, in the form of dental plaque adhered to hard and soft oral tissues. Bacterial biofilm is an extremely important factor in the etiology of dental caries and periodontal diseases, as well as in post-surgical infections 1.
The mouth harbors different microbial species that can be distributed across multiple ecosystems: oral epithelium, dorsum of the tongue, supragingival tooth surface, dental and epithelial subgingival surface. The contact of saliva with these oral tissues explains why saliva has microorganisms from different regions 2,3.
Saliva is a body fluid that is in contact with the entire oral surface. Its characteristics affect the oral microbiota, increasing or decreasing their survivability. Some proteins in saliva may function as a receptor that promotes the adhesion of microorganisms to dental surfaces. In addition, saliva is the primary source of nutrients for about 108 to 109 viable microorganisms/mL 4,5.
Teeth have a mineralized, not scaly surface, which favors the development of large microbial deposits. Although more than 700 bacterial species have been isolated in dental plaque and described, some species remain unidentified 6,7.
Infectious oral diseases are a major risk factor for systemic changes. Dental caries is a multifactorial infectious disease with genetic, environmental, dietary and bacterial causes. A considerable number of studies showed a significant association between Streptococcus mutans and early carious lesions 8-12.
S. mutans is a gram-positive bacterium with high fermentability that, thereby, accumulates a large amount of acids and promotes pH reduction. It is a facultative microorganism capable of producing extracellular and intracellular polysaccharides, which favor microbial adhesion 10. E. faecalis, another important bacterial species investigated in several studies because of its association with endodontic and nosocomial infections 13-15, is a gram-positive facultative species whose virulence is explained by the production of aggregation substances and surface adhesion agents that favor their adhesion to host cells and the extracellular matrix, which facilitates tissue invasion 15. Moreover, the role they play in endodontic infections also depend on their ability to survive in adverse conditions, such as environments with a high pH and high concentrations of sodium chloride 13,16. Pseudomonas aeruginosa, a gram-negative facultative bacterium, is an opportunistic pathogen that rarely causes disease in a healthy immune system, but may exploit any weaknesses of the body to establish infection. This characteristic, together with its natural resistance to a large number of antibiotics and antiseptic agents, explains its high frequency in hospitals 16.
Socransky & Haffajee 17 classified microorganisms as potential or not potential pathogens. A microorganism is classified as a pathogen when it is associated with a disease, found in high numbers in diseased sites, and absent or reduced in sites that demonstrate clinical resolution; moreover, it should induce cellular or humoral immune responses, cause disease in experimental animal models and have virulence factors responsible for the destruction of host tissues.
The oral microbiota is also associated with the presence of bacteria in the blood (bacteremia), a means by which local infections spread to distant organs. Bacteremia is usually transient, because immune responses are vigorous when bacteria are detected in the blood. Infectious endocarditis is a rare but serious infection of the heart valves or heart endothelial surfaces 18. Another critical factor associated with the risk posed by oral microbiota is the handling of oral tissues, which may favor the invasion of bacteria into the bloodstream.
Mouthwashes have been used as antibacterial agents before, during and after surgeries, to reduce microorganisms and prevent their spread. Several substances have been recommended for that purpose. One of them, chlorhexidine, has been widely studied, and its antibacterial effect has been well established. Other substances indicated for oral antisepsis are quaternary ammonium compounds, whose antibacterial effectiveness has also been demonstrated 10,16.
The antibacterial activity of antiseptic solutions available in the market should be analyzed because of the clinical importance of bacteria in common diseases of the mouth and their possible role in other infections. This study evaluated the antibacterial effect of mouthwashes on S. mutans, E. faecalis and P. aeruginosa using agar diffusion and direct exposure tests.
MATERIAL AND METHODS
Biological indicators
For this study, three samples of microorganisms obtained from the American Type Culture Collection were used:
1. Streptococcus mutans (ATCC 25175)
2 . Enterococcus faecalis (ATCC 29212)
3 . Pseudomonas aeruginosa (ATCC 27853)
The experimental method used in this study has been described elsewhere 19. The strains were inoculated into 7 ml of brain heart infusion (BHI, Difco Laboratories, Detroit, MI) and incubated at 37 ° C for 24 hours. The three indicator microorganisms were grown on the surface of brain heart infusion agar (BHIA, Difco Laboratories, Detroit, MI) under the same incubation conditions. Microbial cells were suspended in saline solution to achieve a final concentration of about 3 X 108 cells/mL, adjusted to a no. 1 McFarland standard.
Experimental solutions
The antiseptic solutions tested in this study were 0.07% cetylpyridinium chloride (Oral B Pro Health Clinical Protection ®, The Procter & Gamble Manufacturing Company, 2200 Lower Road, Iowa City, IA), 0.075% cetylpyridinium chloride (Colgate Plax soft mint ®, Colgate-Palmolive Industrial Ltda, V. Anchieta, 14 Km, São Bernardo do Campo, Brazil), 0.12 % chlorhexidine gluconate (Colgate Periogard ®, Colgate-Palmolive Industrial Ltda, V. Anchieta, 14 Km, São Bernardo do Campo, Brazil), and benzalkonium chloride 1.30 mg and lidocaine hydrochloride 25 mg (antiseptic spray, Nexcare®, 3M, Nova Odessa, Brazil).
Agar diffusion test
For the agar diffusion test, 15 Petri dishes with 20 ml BHIA were inoculated with 0.1 mL of the microbial suspension using sterile swabs. The inoculum was spread over the surface of the culture medium to produce confluent growth. Thirty-six 9-mm paper disks were immersed in the experimental solution for one minute. For the negative control, the other three paper discs were immersed in distilled water for one minute and placed on agar plates. Three paper discs were placed on each plate containing culture medium (BHIA). The Petri dishes were kept at room temperature for one hour and then incubated at 37° C for 48 hours. The diameters of the zones of microbial inhibition were measured in the paper disks containing the test substances. Positive and negative controls were inoculated and non-inoculated BHIA plates kept under identical incubation conditions. The entire experiment was performed under aseptic conditions.
Direct exposure test
For the direct exposure test, 216 #50 sterilized absorbent paper points (Tanari, Tanariman Industry Ltda., Manacapuru, Brazil) were immersed in microorganism suspensions for 5 minutes and then placed on Petri dishes and covered with 10 ml of one of the four antiseptic solutions or sterile distilled water (control group). At one, five, 10 and 30 minutes, 3 absorbent paper points were removed, transported individually and immersed in 7 mL of Letheen broth (Difco Laboratories, Detroit, MI ) and incubated at 37° C for 48 hours. After that, an inoculum of 0.1 mL of the Letheen broth was transferred to 7 mL of BHI under identical incubation conditions. Microbial growth was assessed by turbidity of the culture medium. All experiments were performed under aseptic conditions.
RESULTS
Agar diffusion test
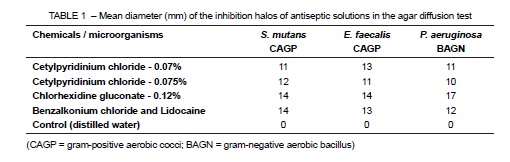
All antiseptic solutions under test had an antimicrobial effect against the biological indicators. The inhibition zones were larger than 10 mm for all substances and microorganisms. Results of the agar diffusion test are shown in Table 1.
Direct exposure test
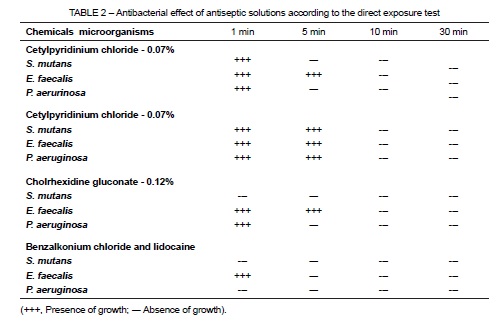
The benzalkonium chloride solution had an antimicrobial effect against all biological indicators at 5 minutes, whereas cetylpyridinium chloride and chlorhexidine gluconate had an antimicrobial effect against all microorganisms only at 10 minutes. The results of the direct exposure test are shown in Table 2.
DISCUSSION
The mouth is an organic environment with a complex microbiota that contains gram-positive and gram-negative bacteria, fungi, protozoa and viruses. These bacteria are distributed, at various concentrations, into different oral ecosystems: oral epithelium, the tongue, supragingival tooth surface, subgingival tooth and epithelial surfaces and saliva. Saliva has no characteristic microbiota, but carries all that is found in the other oral sites 10,19.
Bacterial control under different clinical circumstances, such as pre-operative antiseptic protocols, is essential. The adoption of an aseptic technique during any clinical surgery requires the use of an effective antiseptic, as well as the antisepsis of hands and mouth with effective antimicrobial agents to reduce the microbial population considerably 19. Various antiseptic solutions at different concentrations have been recommended, such as: chlorhexidine gluconate; preparations containing povidone-iodine alcoholic solution of iodine, such as 1% iodine alcohol; 70% isopropyl alcohol; 1% triclosan; cetylpyridinium chloride; benzalkonium chloride. Recent studies have evaluated chlorhexidine, cetylpyridinium chloride and benzalkonium chloride 20-30.
Before discussing our results, the methods used in this study should be analyzed. The spectrum of activity of an antimicrobial agent is essential to improve infection control. In general, three in-vitro techniques stand out: the dilution method, which produces a quantitative result for the antimicrobial agent; the agar diffusion test, which produces a zone of inhibition around the agent; and the direct exposure test, which provides qualitative information about the substance. All techniques have advantages and disadvantages. The dilution method can be used only with substances that are soluble in the culture medium. The size of the microbial inhibition zone depends on the solubility and diffusivity of the test substance in the agar diffusion test and, therefore, cannot express its full potential. The direct exposure test is correlated with the efficacy of substances and their direct contact with microorganisms. This method is independent of other variables and easy to perform in a laboratory 19,31-33. Therefore, this study used more than one test to minimize distortions.
Our methods were chosen after an analysis of previous studies that used them 31-33. The benchmark of the agar diffusion test is the measurement of zones of microbial growth inhibition. Our study did not use solubility and diffusivity of the agents as criteria to select test solutions. Agar concentration, temperature, pH, absence of pre-incubation, culture medium resection and longer times than necessary for a correct analysis are some of the factors that may lead to questionable results. The agar diffusion test, which does not distinguish between bacteriostatic and bactericidal properties of the solutions under evaluation, is widely used in microbiology and has been defined as the standard reference for susceptibility testing. The results of different experiments should be carefully examined and interpreted to avoid unwarranted extrapolations to clinical applications 19,33.
The bacteria selected for this study are microorganisms that play an important role in dental caries, endodontic infections and nosocomial infections, and that have distinct morphological, tinctorial and respiratory characteristics. The basis for the inclusion of microorganisms in this study was the fact that other authors studied them 10,31-33. The culture media, in particular, were those that respond to the nutritional requirements of fastidious microorganisms 33. The time points for measurements were chosen because of their importance in the context of this study.
All experimental techniques were chosen because they are simple, reproducible and effective for the purposes outlined in this study. Moreover, they are suitable to detect different types of facultative anaerobic bacteria and to recover small quantities of microbial cells, especially when studying rich microbial ecosystems. Parameters, such as the aseptic technique, were strictly followed during the performance of tests and application of the study methods.
The bacteria used were selected because of their clinical importance. Dental caries is an infectious disease of the mouth in which there is an important participation of S. mutans, a species whose potential adhesion may define its importance in dentistry 5,8-12. E. faecalis, a gram-positive facultative bacterium, is an important pathogenic agent in nosocomial and endodontic infections 13-16,34-36. Love 34 found a possible mechanism that might explain how E. faecalis bacteria survive and grow inside the dentinal tubules and re-infect root canals after filling. The virulence of E. faecalis, which may be responsible for endodontic treatment failure, may be associated with their capacity to invade dentinal tubules and adhere to collagen in the presence of human serum. Pseudomonas aeruginosa, a gram-negative facultative species, has also been associated with nosocomial infections 16.
The oral antiseptic solutions tested in this study had an antibacterial effect against Streptococcus mutans, Enterococcus faecalis and Pseudomonas aeruginosa. In the agar diffusion test, 0.07% and 0.075% cetylpyridinium chloride had bacterial inhibition halos measuring at least 10 mm, whereas 0.13% benzalkonium chloride halos were greater than 12 mm for the biological indicators under test. The largest halos in the agar diffusion test were found for 0.12 % chlorhexidine gluconate (greater than 14 mm). The direct exposure test showed that 0.07% and 0.075% cetylpyridinium chloride and 0.12% chlorhexidine gluconate had an antibacterial effect against all microorganisms only at 10 minutes of direct exposure to the bacteria. The benzalkonium chloride solution had an antibacterial effect against biological indicators at 5 minutes.
Mouthwashes are recommended for the control of bacterial microbiota, but not all dentists recognize the importance of their use. Antibacterial mouthwashes are effective in reducing planktonic microorganisms and controlling plaque and gingivitis.
The results of this study are in agreement with those reported in two reviews 20,37. Lawrence 20 examined the antimicrobial activity of chlorhexidine, benzalkonium chloride, povidone-iodine and phenol. Chlorhexidine had a greater antimicrobial activity than the derivatives of quaternary ammonium. Hennessey 37 evaluated the antimicrobial properties of chlorhexidine, confirmed its effectiveness and found that gram-positive microorganisms were more sensitive than gram-negative ones, and that staphylococci were more resistant than streptococci.
Chlorhexidine is a cationic agent that has an antibacterial effect. The cationic nature of this compound promotes its connection with the anionic group (phosphate groups of teichoic acids in gram-positive bacteria and lipopolysaccharide in gramnegative bacteria) on the bacterial surface and change it entirely. Potassium ions are the first to appear when the cytoplasmic membrane promotes the precipitation of cytoplasmic proteins by disturbing the osmotic balance of the cell and affecting metabolism, growth and cell division due to the inhibition of the ATPase membrane and the anaerobic process 32.
Estrela et al. 32 described the minimum concentrations of 1% sodium hypochlorite and 2% chlorhexidine to inhibit S. aureus, E. faecalis, P. aeruginosa, B. subtilis, C. albicans and a mixture of these microorganisms from a dilution series of 1:10. Their results showed that the minimum inhibitory concentration of sodium hypochlorite was 1% for S. aureus, E. faecalis, P. aeruginosa and C. albicans, 0.1% for B. subtilis and 1% for the mixture. The solution of 2% chlorhexidine had a minimum inhibitory concentration of 0.000002 % for S. aureus, 0.002 % for P. aeruginosa, and 0.02% for E. faecalis, B. subtilis, C. albicans and the mixture.
Cationic quaternary ammonium compounds have antimicrobial properties and are stable and soluble in water. Their antibacterial activity is associated with the positive charge of the molecule (cation). The structures of the quaternary ammonium compounds are related to ammonium chloride (NH4Cl) 24. The cationic part of the molecule stimulates binding to the anionic compound on the surface of bacteria and may affect the integrity of the cytoplasmic membrane. Once the cytoplasmic membrane is damaged, the functions that involve membrane permeability are affected. The inactivation of the cytoplasmic membrane enzymes has serious consequences, such as protein denaturation 27,28,36,38. Cationic detergents (quaternary ammonium compounds) are widely used as surface active agents. Two of these detergents are well known: benzalkonium chloride and cetylpyridinium chloride 27.
Haps et al. 26 conducted a systematic review of studies about cetylpyridinium chloride in mouthwashes used as adjuncts to tooth brushing and their effect on the prevention of plaque accumulation and gingival inflammation. Independent screening of titles and abstracts of 3250 publications revealed that eight studies met eligibility criteria. Descriptive comparisons were made for brushing only or brushing and using mouthwash. Evidence showed that cetylpyridinium chloride in mouthwashes used as adjuvant or unsupervised oral hygiene provides additional benefits in reducing plaque accumulation and gingival inflammation. According to Osso et al. 30, most studies show that mouthwashes containing chlorhexidine or essential oils and methyl salicylate provide clinical benefits to prevent plaque and gingivitis. Studies found that chlorhexidine, essential oils and cetylpyridinium chloride are effective and confirmed the efficacy of antiseptic mouthwashes in reducing plaque and gingivitis. However, current evidence is insufficient to support the claim that mouthwashes may reduce the risk of developing periodontitis or the rate of progression of this disease. Estrela et al. 36 confirmed the antimicrobial activity of cetylpyridinium chloride in 40 root canals infected by E. faecalis for 60 days. Their results showed that E. faecalis was still present after cleaning and shaping. Cetylpyridinium chloride reduced the number of bacteria and, in the agar diffusion test, inhibited microbial growth. These results were similar to those of 2% chlorhexidine and better than those of 2.5% sodium hypochlorite, which confirmed the antimicrobial effect of cetylpyridinium chloride against endodontic infections by E. faecalis.
Antisepsis before oral surgeries should be a routine for maxillofacial surgeons, as the control of the microbiota in the oral cavity may promote tissue repair.
CONCLUSIONS
The antiseptic solutions under study here had an antibacterial effect when in direct contact with S. mutans, E. faecalis and P. aeruginosa.
REFERENCES
1. Timmerman MF, van der Weijden GA. Risk factors of periodontitis. Int J Dent Hyg. 2006;4(1):2-7. [ Links ]
2. Shearer BG. Biofilm and the dental office. J Am Dent Assoc. 1996;127:181-9.
3. Spratt DA. Significance of bacterial identification by molecular biology methods. Endod Top. 2004;9:5-14.
4. Schonfeld SE. Oral microbial ecology. In: Slots J, Taubman MA. Contemporary oral microbiology and immunology. St. Louis: Mosby, 1992. p. 267-74.
5. Marsh PD. Plaque as a biofilm: pharmacological principles of drug delivery and action in the sub- and supragingival environment. Oral Dis. 2003;9:16-22.
6. Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontology. 2002;28:12-55.
7. Socransky SS, Haffajee AD. Microbiologia da doença periodontal. In: Lindhe J, Karring T, Lang NP. Tratado de periodontia clínica e implantodontia oral. 4ª ed. Rio de Janeiro: Guanabara Koogan; 2005. p. 105-47.
8. Curtiss RIII. Genetic analysis of Streptococcus mutans virulence. Curr Top Microbiol Immunol. 1985;118:253-77.
9. Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353-80.
10. Loesche WJ. Cárie dental – Uma infecção tratável. Rio de Janeiro: Cultura Médica; 1993.
11. Caufield PW, Walker TM. Genetic diversity within Streptococcus mutans evident from chromosomal DNA restriction fragment polymorphisms. J Clin Microbiol. 1989;27:274-6.
12. Matsumoto Y, Sugihara N, Kosek M, Maki Y. A rapid and quantitative detection system for Streptococcus mutans in saliva using monoclonal antibodies. Caries Res. 2006;40:15-9.
13. Molander A, Reit C, Dahlén G, Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J. 1998;31:1-7.
14. Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative retreatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:86-93.
15. Portenier I, Waltimo T, Haapasalo M. Enterococcus faecalis – The root canal survivor and "star" in post-treatment disease. Endod Top. 2003;6:135-59.
16. Slots J, Taubman MA. Contemporary oral microbiology and immunology. St Louis: Mosby; 1992.
17. Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322-31.
18. Villoria GEM, Costinha LHC. Antissépticos bucais no controle da bacteremia de origem oral. Rev HUPE. 2013;12:76-83.
19. Bammann LL, Estrela C. Microbiological aspects in endodontics. In: Estrela C. Endodntic science. 2ª ed. Artes Médicas: São Paulo; 2009.
20. Lawrence CA. Antimicrobial activity in vitro of chlorhexidine. J Am Pharmac Assoc. 1960;49:731-4.
21. Schroeder HE, Marthaler TM, Muhlemann HR. Effects of some potencial inhibitors on early calculus formation. Helv Odont Acta. 1962;6:6-9.
22. Gjermo P, Baastad KL, Rölla G. The plaque-inhibiting capacity of 11 antibacterial compounds. J Periodont Res. 1970;5:102-9.
23. Bonesvoll P, Gjermo P. A comparison between chlorhexidine and some quaternary ammonium compounds with regard to retention, salivary concentration and plaqueinhibiting effect in the human mouth after mouth rinses. Archs Oral Biol. 1978;23:289-94.
24. Pelczar-Jr MJ, Chan ECS, Krieg NR. Microbiology: concepts and applications. New York: McGraw-Hill; 1993. p. 210-28.
25. Quirynen M, Gizani S, Mongardini C, Declerck D, Vinckier F, van Steenberghe D. The effect of periodontal therapy on the number of cariogenic bacteria in different intraoral niches. J Clin Periodontol. 1999;26:322-7.
26. Haps S, Slot DE, Berchier CE, Van der Weijden GA. The effect of cetylpyridinium chloride-containing mouth rinses as adjuncts to tooth brushing on plaque and parameters of gingival inflammation: a systematic review. Int J Dent Hyg. 2008;6:290-303.
27. Busscher HJ, White DJ, Atema-Smit J, Geertsema-Doornbusch G, De Vries J, Van Der Mei HC. Surfactive and antibacterial activity of cetylpyridinium chloride formulations in vitro and in vivo. J Clin Periodontol. 2008;35:547-54.
28. Tortora GJ, Funke BR, Case CL. Microbiology: an introduction. 10th ed. San Francisco: Benjamin Cummings; 2009. 960p.
29. Alves D, Costa AL, Almeida RF, Carvalho JFC, Felino A. Cetylpyridinium chloride – a literature review. Rev Port Estomatol Med Dent Cir Maxilofac. 2012:53:81-9.
30. Osso D, Kanani N. Antiseptic mouth rinses: an update on comparative effectiveness, risks and recommendations. J Dent Hyg. 2013;87:10-8.
31. Estrela C, Estrela CRA, Pécora JD, Amorim LFG, Toledo OA Eficácia antomicrobiana de formulações de digluconato de clorexidina de concentrações e procedências diferentes. Robrac. 2004;13:10-3.
32. Estrela C, Estrela CRA. Controle de infecção em odontologia. São Paulo: Artes Médicas; 2003. 188p.
33. Estrela C, Pimenta FC, Estrela CRA. Testes microbiológicos aplicados à pesquisa. In: Estrela C. Metodologia científica. São Paulo: Artes Médicas; 2005. p. 295-326.
34. Love RM. Enterococcus faecalis: mechanism for its role in endodontic failure. Int Endod J. 2001;34:399-406.
35. Estrela C, Alencar AHG, Decurcio DA, Borges AH, Guedes AO, Estrela CRA. Influência de estratégias de sanificação no sucesso do tratamento da periodontite apical. Robrac. 2012;21:367-75.
36. Estrela C, Sousa-Neto MD, Alves DRS, Alencar AHG, Santos TO, Pécora JD. A preliminary study of the antibacterial potential of cetylpyridinium chloride in root canals infected by E. faecalis. Braz Dent J. 2012;23:645-53.
37. Hennessey TS. Some antibacterial properties of chlohexidine. J Periodontol. 1973;12:61-7. 38. Estrela C, Pécora JD. Root canal irrigants. In: Estrela C. Endodontic science. 2nd ed. São Paulo: Artes Médicas; 2009. p. 697-744.
 Correspondence:
Correspondence:
Marcel da Silva Garrote
Department of Endodontics, School of Dentistry, Universidade Federal de Goiás
Av. Universitária esquina com Primeira Avenida, s/n, Setor Leste Universitário
CEP 74605-220, Goiânia, GO, Brazil
E-mail: estrela3@terra.com.br













