Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Stomatos
versão impressa ISSN 1519-4442
Stomatos vol.21 no.41 Canoas Jul./Dez. 2015
Effect of raloxifene on alveolar bone resorption after mucoperiosteal flap surgery in mice
Efeito do raloxifeno sobre reabsorção óssea alveolar após cirurgia de retalho mucoperiosteal em camundongos
Cristiane Alencar I; Dalva Maria Pereira Padilha II; Luis Carlos da Fontoura Frasca II; Daiane Cerutti-Kopplin III; Sabrina Rebollo Zani IV; Elken Gomes RivaldoV
I MSc from the Graduate Program in Dentistry, Universidade Luterana do Brasil (ULBRA), Canoas, RS, Brazil
II MSc and PhD, and professors at Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil
III professor at Universidade Regional Integrada do Alto Uruguai e das Missões, RS, Brazil
IV PhD in Dental Prosthetics
V professor at the Graduate Program in Dentistry, ULBRA, Canoas, RS, Brazil
The authors have no conflicts of interest to declare concerning the publication of this manuscript
ABSTRACT
Background: Mucoperiosteal flap surgeries (MFS) are carried out to provide access to the alveolar bone and root surfaces in several clinical situations. Nevertheless, they lead to a variable degree of alveolar bone resorption. Raloxifene is an agonist in bone, and acts inhibiting bone loss. Objective: To evaluate the effect of raloxifene in preventing alveolar bone resorption after MFS using an experimental model of mouse mandibles. Methods: MFS was performed on the buccal aspect of the left side of the mandible (BL) in 20 male CF1 Musdomesticus mice divided into two groups with the same number of animals: the experimental group was treated once daily with raloxifene injections (3 mg/kg), and the placebo group was treated with daily injections of the vehicle. The buccal aspects of right hemimandibles were used as controls (BR). Mandibles were removed, defleshed and stained with toluidine blue in a stereomicroscope. Digital images were obtained and the alveolar bone loss was measured (mm²) using an image analysis software. Results: The BL area exhibited significantly more bone loss (Student t test; p < 0.01) when compared to the BR area, in both groups. No statistically significant difference was observed between the experimental and the placebo groups. Conclusion: In this study, raloxifene did not inhibit alveolar bone resorption following MFS in male mice.
Keywords: Raloxifene Hydrochoride; Alveolar Bone Loss; Surgery, Oral.
RESUMO
Introdução: Cirurgias de retalho mucoperiosteal (CRM) são realizadas para permitir acesso ao osso alveolar e à superfície radicular em várias situações clínicas. No entanto, elas levam a um grau variável de reabsorção óssea alveolar. O raloxifeno tem ação agonista em tecido ósseo e atua inibindo perda óssea. Objetivo: Avaliar o efeito do raloxifeno na prevenção de reabsorção óssea alveolar após CRM usando o modelo experimental de mandíbulas de camundongos. Métodos: Foram realizadas CRMs na face vestibular, lado esquerdo, das mandíbulas (VE) de 20 camundongos CF1 Musdomesticus machos, divididos em dois grupos com mesmo número de animais: o grupo experimental foi tratado uma vez ao dia com injeções de raloxifeno (3 mg/kg), e o grupo placebo foi tratado uma vez ao dia com injeções do veículo. A face vestibular do lado direito da hemimandíbula foi usada como controle (BD). As mandíbulas foram removidas, dissecadas e coradas com azul de toluidina sob um estereomicroscópio. Imagens digitais foram obtidas e a perda óssea alveolar foi medida (mm²) usando um software de análise de imagens. Resultados: A área VE exibiu perda óssea significativamente maior (teste t de Student; p < 0,01) quando comparada com a área BD, em ambos os grupos. Não foi observada diferença estatisticamente significativa entre os grupos experimental e placebo. Conclusão: Neste estudo, o raloxifeno não inibiu a reabsorção óssea alveolar após CRM em camundongos machos.
Palavras-chave: Cloridrato de Raloxifeno; Perda do Osso Alveolar; Cirurgia, Oral.
INTRODUCTION
Bone is a dynamic tissue with high remodeling capacity in response to the body's metabolic requirements. Under normal circumstances, bone volume and mass are held constant by bone resorption and apposition processes 1. Nevertheless, some factors, including surgical bone exposure, may lead to some imbalance in these mechanisms.
In dentistry, surgical access to the alveolar bone is routinely conducted as part of different treatment approaches, including restoration, periodontal treatment, rehabilitation, endodontic treatment, and in the surgical removal of teeth and/or pathological processes. During the dissection process, the periosteum is detached from the alveolar bone, leading to a resorptive phase due to the stimulation offered by osteoclastic activity and loss of bone crest 2-4. In rodent mandibles, resorption is also observed after surgery 4,5.
Alveolar bone volume reduction may interfere with the success of oral rehabilitation treatments. Therefore, loss of this bone tissue should be prevented 6. The use of systemic or local drugs in the prevention of bone resorption has been studied using animal models 7-16.
Raloxifene, a selective estrogen receptor modulator, is an agonist to the human skeleton. The compound has been shown to act as an antiresorption drug by reducing biochemical markers of bone remodeling and preserving bone mineral density in the lumbar spine and femur neck, with significant reduction in the occurrence of new fractures. In addition, raloxifene is an antagonist to endometrial and mammary tissues 17,18. The preventive effect of raloxifene on the expression of selective bone turnover markers has also been observed during the alveolar healing process in female rats submitted to ovariectomy surgery 15,19,20.
This study aims to evaluate the effect of raloxifene in preventing alveolar bone resorption after mucoperiosteal flap surgery (MFS) using an experimental model of mouse mandibles 5.
MATERIALS AND METHODS
This study was approved by the Research Ethics Committee of Universidade Luterana do Brasil (ULBRA).
A total of 20 3-month-old male CF1 mice (Musdomesticus) weighing 30 g on average were used in this study. Animals were obtained from the colonies maintained by state-owned research institution Fundação Estadual de Produção e Pesquisa em Saúde. Mice were randomly divided into two experimental groups, consisting of 10 mice each, all undergoing MFS. The first group (experimental group, EG) was treated with raloxifene injections; the second group (placebo group, PG) was given injections of the vehicle used in raloxifene dilutions.
Animals were maintained in individual sterilized plastic cages (Beira Mar, São Paulo, SP, Brazil) with an iron cover and solid bottom during the experiment. Sterilized bedding (Vet-Sul, Porto Alegre, RS, Brazil) was provided. Mice consumed standard food (Nutrival, CR-1, Nutrival Nutrients, Curitiba, PR, Brazil) and distilled water ad libitum. Standard conditions of light (12-hour light/12-hour dark cycle) and temperature (20°C) were kept during the experiment. The experiment was conducted under proper ventilation and all animals were monitored daily.
Experimental procedure
Initially, mice of both groups were weighed and anesthetized using intramuscular ketamine 100 g/L (Dopalen, Agribrands do Brazil, Paulina, Brazil) + 2% aqueous solution of 2-(2,6-xilidine)-5,6-dihydro-4-H-1,3-thiazine hydrochloride (Rompun, Bayer S.A., São Paulo, Brazil) at a 1:1 ratio in a dosage of 1.0 mL/kg. The mucosa was separated from the underlying bone after an incision at the marginal gingiva with a small elevator and then immediately readapted without any suture 4,5,7,10-13. The mucoperiosteal flap was performed on the buccal aspect of the left side of the lower left molars in a procedure that took approximately 40 seconds; the right side was used as control (CG). Animals received only water during the initial 24 hours, to avoid displacement of the flap.
Mice in the EG were treated with subcutaneous injections of raloxifene (Raloxifenchloridrate, Eli Lilly, Brazil) (3mg/kg) dissolved in 100 μL of olive oil, daily, throughout the experimental period. The PG received the vehicle (100 μL olive oil/mice) (Figueira da Foz, Portugal), also daily 8,10. Animals were weighed daily before the injections to calculate the correct dose. Twenty-one days after the surgical procedure, animals were euthanized by means of cervical dislocation 4 under anesthesia.
Under a surgical stereomicroscope (M900, D F Vasconcelos, São Paulo, Brazil), mandibles were sectioned in the midline, defleshed, and all the organic material was removed with the help of sodium hypochlorite (Biodinâmica Química e Farmacêutica Ltda., Ibiporã, Brazil). Specimens were then stored in 10% buffered formalin for 12 hours 21-23. Each hemimandible was stained with 1% toluidine blue to disclose the root-exposed area. Limits of enamel, cementum and evident bone were observed in a stereomicroscope (Stemi SV6, Carl Zeiss, Inc., USA) under magnification (x3.2), using standardized position 24 and light. Images were digitally captured (Pixera Professional, Pixera, San Jose, USA).
Bone loss analysis
Bone loss was measured by a single blind, calibrated examiner using image analysis software (Image Tool, UTHSCA, Texas, USA). Two independent measurements were conducted with an interval of 1 week between them, in order to allow reproducibility analysis. In the present study, intra-examiner and trans-experiment reproducibility values (95% confidence interval) were represented by the means of differences between the pairs of measurements of alveolar bone loss (ABL), and ranged between -0.03 mm2 and 0.03 mm2.
In the buccal aspect, the area of the ABL was measured in the first molar, whereas in the lingual aspect, the area of ABL was measured in the first and second molars using the reference points proposed by Tatakis and Guglielmoni 23 and modified by Hilgert et al. 25. The ABL area in the lingual aspect was defined as follows: mesially, by the mesial edge (cementoenamel junction [CEJ] to the alveolar bone) of the mesial root of the first molar; distally, by the distal edge (CEJ to the alveolar bone) of the distal root of the second molar; coronally, by three points on each of the two molar teeth (two points defined by the position of the CEJ on the mesial and distal aspects of the tooth and one defined by the most apical position of the CEJ on the tooth surface); apically, by the most apical position of the alveolar bone on the first and second root surfaces (Figure 1). Results are presented in square millimeters (mm²).

Statistical analysis
The Student t test for independent samples was used to assess the presence of significant differences in respect to alveolar bone loss between mice treated with raloxifene and control mice. Furthermore, the Student t test was also used to check for intragroup differences (e.g., in BL and BR ABL areas of mice within each group). The value set to reject the null hypothesis was p ≤ 0.05. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 12.0 for Windows®.
RESULTS
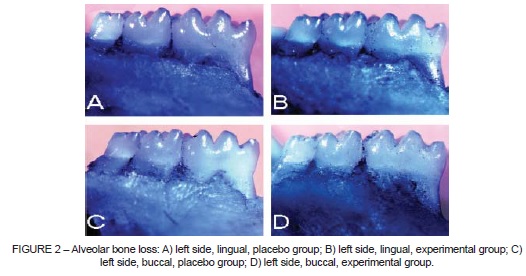
Bone resorption was observed 3 weeks after MFS. ABL was significantly greater in the operated area (BL) (Student t test; p < 0.01) when compared to the control side (BR) (Table 1).
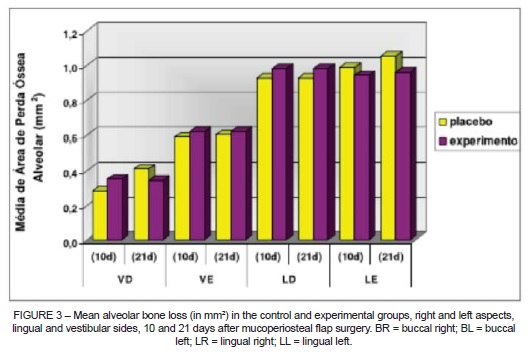
Bone loss did not vary significantly when EG and CG were compared (Figure 2). Bone loss was observed (CG vs. EG, respectively) in BL: 0.60±0.23 mm2 vs. 0.62±0.39 mm2 (p = 0.91); in BR: 0.41±0.17 mm2 vs. 0.34±0.09 mm2 (p=0.69); in LL: 1.05±0.27 mm2 vs. 0.96±0.28 mm2 (p=0.47); and in LR: 0.93±0.26 mm2 vs. 0.98±0.18 mm2 (p=0.59) (Figure 3).
No weight variation was observed in animals throughout the experimental period.
DISCUSSION
The present study evaluated the effect of raloxifene in terms of inhibiting ABL after MFS in mice. Under the experimental circumstances here described, raloxifene was not able to prevent or even reduce bone resorption. To our knowledge, this is one of the first studies to assess whether raloxifene affects bone resorption using a rodent model in male mice.
In the present study, an experimental model of MFS was used to induce ABL in mouse mandibles, as different studies have reported bone resorption due to bone exposure by flap displacement 3,5,7. Yaffe et al. 4 conducted a study with rats and observed considerable bone remodeling after MFS in maxillary bones. In mice, this process is sex-independent 5. These studies are in agreement with the results shown in the present experiment, as ABL was significantly greater in the operated side (BL) when compared to the control side (BR).
Bone resorption may interfere negatively with the success of oral rehabilitation therapies. Therefore, the search for drugs and treatments that can prevent or minimize ABL is an important field of periodontal research. Raloxifene, a selective estrogen receptor modulator used in osteoporosis treatment and prophylaxis was tested for its role in bone resorption. The drug mimics the effects of estrogen in the bone, with the added benefits of not stimulating the uterine endometrium and reducing the risk of breast cancer and the incidence of coronary events and stroke 26-28. In vitro, raloxifene plays a role in the modulation of bone homeostasis by inhibiting osteoclastogenesis and bone resorption, stimulating osteoblast activity, and inhibiting interleukin 1β, interleukin 6 and TNF-α 29. In vivo studies also demonstrate positive effects of the drug on the maintenance of mineral bone density of the lumbar spine, femoral neck 17,18,28 and tibia 30. Those studies also reported a decrease in biochemical markers of bone remodeling, such as osteocalcin, alkaline phosphatase, interleukin 1β, and interleukin 6 17,18, as well as a reduced risk of microfractures 28. There is evidence that the efficacy of raloxifene is similar in the bones of male rats and ovariectomized female rats 15,31, with an increased expression of selective bone turnover markers in ovariectomized female rats during the alveolar healing process 20. Conversely, raloxifene replacement has proved unable to recover the estrogen-deficient states 20,32. Thus, the action of the drug does not depend on sex.
Even though the effects of raloxifene in bone metabolism have been widely recognized, the results obtained in the present study have shown that the drug does not prevent MFL-induced ABL. This result may be explained by the evidence suggesting that bone mineral density of spine and hip bones cannot be used as predictors of maxillary bone density 33,34. The hypothesis of site-specific bone remodeling formulated by Podenphant and Engel 35 may also help interpret the results of the present study. Those authors investigated 24 different sites of the skeleton and observed that bone formation undergoes considerable regional variations. This hypothesis was confirmed by Verna et al. 36, who compared the parameters of bone remodeling of the mandible with those of the iliac crest. Titanium implants represent a good example of site specificity in bone remodeling, with data showing that 76% of failure in orthopedic implants occur in patients with osteoporosis 37, while the risk of failure in dental implants does not seem to be higher for patients with osteoporosis 38.
The effects of other drugs, such as bifosfonates and doxycycline, in ABL have been investigated previously. Bifosfonates, drugs prescribed in the treatment and prevention of osteoporosis, have been shown by several studies to decrease ABL after MFS in rats 4,10,12. The results found by Yaffe et al. 4,12 demonstrate a decrease in ABL after bifosfonate therapy. Doxycycline was also shown to be able to prevent MFS-induced ABL in a rat model, according to Grevstad 9. Nevertheless, it is important to carefully consider the methodology adopted in the evaluation of ABL in each study.
Different methods have been proposed to study ABL in animal models, with some methodologies evaluating the mineralized matrix 12-14,39,40, others evaluating histological sections 9,10, and others evaluating linear distance measurements 39-41. Yet, all these methods were shown to have limitations 12,39,40. Yaffe et al. 14 demonstrated the effect of alendronate on preventing ABL using high resolution X-ray microradiography analysis. However, no significant differences were observed when ABL was assessed using measurements of the exposed root area.
Further studies are needed to assess the effect of raloxifene in mandibular bones in terms of bone mineral density, decrease in bone turnover biomarkers, or positive changes in cell counts. Further knowledge of these aspects is relevant for the aim of maintaining alveolar bone.
REFERENCES
1. Marx RE, Garg AK. Bone structure, metabolism, and physiology: its impact on dental implantology. Implant Dent. 1998;7:267-76. [ Links ]
2. Ramfjord SF, Costich ER. Healing after exposure of periosteum on the alveolar process. J Periodontol. 1968;39:199-207.
3. Staffileno H, Levy S, Gargiulo A. Histologic study of cellular mobilization and repair following a periosteal retention operation via split thickness mucogingival flap surgery. J Periodontol. 1966;37:117-31.
4. Yaffe A, Fine N, Binderman I. Regional accelerated phenomenon in the mandible following mucoperiosteal flap surgery. J Periodontol. 1994;65:79-83.
5. Rivaldo EG, Padilha DP, Hugo FN. Alveolar bone loss and aging: a model for the study in mice. J Periodontol. 2005;76:1966-71.
6. Atwood DA. Some clinical factors related to rate of resorption of residual ridges. 1962. J Prosthet Dent. 2001;86:119-25.
7. Binderman I, Adut M, Zohar R, Bahar H, Faibish D, Yaffe A. Alveolar bone resorption following coronal versus apical approach in a mucoperiosteal flap surgery procedure in the rat mandible. J Periodontol. 2001;72:1348-53.
8. Erlandsson MC, Jonsson CA, Lindberg MK, Ohlsson C, Carlsten H. Raloxifene- and estradiol-mediated effects on uterus, bone and B lymphocytes in mice. J Endocrinol. 2002;175:319-27.
9. Grevstad HJ. Doxycycline prevents root resorption and alveolar bone loss in rats after periodontal surgery. Scand J Dent Res. 1993;101:287-91.
10. Kaynak D, Meffert R, Bostanci H, Gunhan O, Ozkaya OG. A histopathological investigation on the effect of systemic administration of the bisphosphonate alendronate on resorptive phase following mucoperiosteal flap surgery in the rat mandible. J Periodontol. 2003;74:1348-54.
11. Seto H, Ohba H, Tokunaga K, Hama H, Horibe M, Nagata T. Topical administration of simvastatin recovers alveolar bone loss in rats. J Periodontal Res. 2008;43:261-7.
12. Yaffe A, Fine N, Alt I, Binderman I. The effect of bisphosphonate on alveolar bone resorption following mucoperiosteal flap surgery in the mandible of rats. J Periodontol. 1995;66:999-1003.
13. Yaffe A, Golomb G, Breuer E, Binderman I. The effect of topical delivery of novel bisacylphosphonates in reducing alveolar bone loss in the rat model. J Periodontol. 2000;71:1607-12.
14. Yaffe A, Iztkovich M, Earon Y, Alt I, Lilov R, Binderman I. Local delivery of an amino bisphosphonate prevents the resorptive phase of alveolar bone following mucoperiosteal flap surgery in rats. J Periodontol. 1997;68:884-9.
15. Ramalho-Ferreira G, Faverani LP, Prado FB, Garcia IR Jr., Okamoto R. Raloxifene enhances peri-implant bone healing in osteoporotic rats. Int J Oral Maxillofac Surg. 2015;44:798-805.
16. Kangas L, Harkonen P, Vaananen K, Peng Z. Effects of the selective estrogen receptor modulator ospemifene on bone in rats. Horm Metab Res. 2014;46:27-35.
17. Morii H, Ohashi Y, Taketani Y, Fukunaga M, Nakamura T, Itabashi A, et al. Effect of raloxifene on bone mineral density and biochemical markers of bone turnover in Japanese postmenopausal women with osteoporosis: results from a randomized placebo-controlled trial. Osteoporos Int. 2003;14:793-800.
18. Weinstein RS, Parfitt AM, Marcus R, Greenwald M, Crans G, Muchmore DB. Effects of raloxifene, hormone replacement therapy, and placebo on bone turnover in postmenopausal women. Osteoporos Int. 2003;14:814-22.
19. Luvizuto ER, Dias SM, Queiroz TP, Okamoto T, Garcia IR Jr., Okamoto R, et al. Osteocalcin immunolabeling during the alveolar healing process in ovariectomized rats treated with estrogen or raloxifene. Bone. 2010;46:1021-9.
20. Luvizuto ER, Dias SS, Okamoto T, Dornelles RC, Okamoto R. Raloxifene therapy inhibits osteoclastogenesis during the alveolar healing process in rats. Arch Oral Biol. 2011;56:984-90.
21. Al-Rasheed A, Scheerens H, Rennick DM, Fletcher HM, Tatakis DN. Accelerated alveolar bone loss in mice lacking interleukin-10. J Dent Res. 2003;82:632-5.
22. May NY, Tatakis DN. Accelerated alveolar bone loss in male HLA-B27 transgenic rats: adult onset. J Periodontal Res. 2004;39:33-6.
23. Tatakis DN, Guglielmoni P. HLA-B27 transgenic rats are susceptible to accelerated alveolar bone loss. J Periodontol. 2000;71:1395-400.
24. Rivaldo EG, Padilha DM, Hugo FN, Hilgert JB, Rybu BR. Reproducibility of a hemi mandible positioning device and a method for measuring alveolar bone loss area in mice. J Oral Sci. 2007;49:13-7.
25. Hilgert JB, Hugo FN, Bozzetti MC, Padilha DM. Comparison of two techniques for measuring alveolar bone loss in mice. Braz Oral Res. 2002;16-s:216.
26. Black LJ, Sato M, Rowley ER, Magee DE, Bekele A, Williams DC, et al. Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest. 1994;93:63-9.
27. Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-97.
28. Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637-45.
29. Taranta A, Brama M, Teti A, De Luca V, Scandurra R, Spera G, et al. The selective estrogen receptor modulator raloxifene regulates osteoclast and osteoblast activity in vitro. Bone. 2002;30:368-76.
30. Evans G, Bryant HU, Magee D, Sato M, Turner RT. The effects of raloxifene on tibia histomorphometry in ovariectomized rats. Endocrinology. 1994;134:2283-8.
31. Folwarczna J, Sliwinski L, Cegiela U, Pytlik M, Kaczmarczyk-Sedlak I, Nowinska B, et al. Raloxifene similarly affects the skeletal system of male and ovariectomized female rats. Pharmacol Rep. 2007;59:349-58.
32. Luvizuto ER, Queiroz TP, Dias SM, Okamoto T, Dornelles RC, Garcia IR Jr., et al. Histomorphometric analysis and immunolocalization of RANKL and OPG during the alveolar healing process in female ovariectomized rats treated with oestrogen or raloxifene. Arch Oral Biol. 2010;55:52-9.
33. Drage NA, Palmer RM, Blake G, Wilson R, Crane F, Fogelman I. A comparison of bone mineral density in the spine, hip and jaws of edentulous subjects. Clin Oral Implants Res. 2007;18:496-500.
34. Lindh C, Obrant K, Petersson A. Maxillary bone mineral density and its relationship to the bone mineral density of the lumbar spine and hip. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:102-9.
35. Podenphant J, Engel U. Regional variations in histomorphometric bone dynamics from the skeleton of an osteoporotic woman. Calcif Tissue Int. 1987;40:184-8.
36. Verna C, Melsen B, Melsen F. Differences in static cortical bone remodeling parameters in human mandible and iliac crest. Bone. 1999;25:577-83.
37. Barrios C, Brostrom LA, Stark A, Walheim G. Healing complications after internal fixation of trochanteric hip fractures: the prognostic value of osteoporosis. J Orthop Trauma. 1993;7:438-42.
38. Garg AK, Winkler S, Bakaeen LG, Mekayarajjananonth T. Dental implants and the geriatric patient. Implant Dent. 1997;6:168-73.
39. Frost HM. Some effects of basic multicellular unit-based remodelling on photon absorptiometry of trabecular bone. Bone Miner. 1989;7:47-65.
40. Kuhr A, Popa-Wagner A, Schmoll H, Schwahn C, Kocher T. Observations on experimental marginal periodontitis in rats. J Periodontal Res. 2004;39:101-6.
41. Menezes AM, Rocha FA, Chaves HV, Carvalho CB, Ribeiro RA, Brito GA. Effect of sodium alendronate on alveolar bone resorption in experimental periodontitis in rats. J Periodontol. 2005;76:1901-9.
 Correspondence:
Correspondence:
Elken Gomes Rivaldo
Rua Pedro de Oliveira Bittencourt, 207
Bairro Tristeza, CEP 91900-230
Porto Alegre, RS, Brazil
e-mail: elkenrivaldo@gmail.com













