Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Stomatos
versão impressa ISSN 1519-4442
Stomatos vol.22 no.42 Canoas Jan./Jun. 2016
Isolation and characterization of dental pulp stem cells from permanent third molars
Isolamento e caracterização de células-tronco pulpares derivadas de terceiros molares permanentes
Catiane Telles I; Alessandra Dutra da Silva II; André Wiltgen II; Márcia Kijner II; Melissa Camassola III; Paulo de Borba II
I dental surgeon from Universidade Luterana do Brasil (ULBRA), Canoas, RS, Brazil
II PhD and adjunct professors at ULBRA, Canoas, RS, Brazil
III professor and researcher at the Graduate Program in Molecular Genetic Diagnosis at ULBRA, Canoas, RS, Brazil
The authors have no conflicts of interest to declare concerning the publication of this manuscript.
ABSTRACT
Objective: To characterize pulp stem cells and evaluate their capacity for expansion and differentiation in vitro. Methods: Pulp tissue was collected from permanent third molars and digested and then the cells were seeded onto plates containing HDMEM medium. Expansion of the cells was performed and then differentiation tests were conducted with osteogenic, chondrogenic and adipogenic induction media; followed by determination of immunophenotypical profiles using specific antibodies with assessment by fl ow cytometry. Results: It was observed that the pulp stem cells exhibited the capacity to adhere to plastic and a high rate of expansion and, after detection with specific stains, it was shown that the cells were capable of differentiation into osteoblasts and chondroblasts, but not into adipocytes. Analysis of the cellular phenotype showed that the cells were negative for CD45, CD69, CD117 and HLADR, and positive for CD13, CD44, CD73, CD90 and CD105. Conclusions: The cells isolated from dental pulp exhibited characteristics compatible with those expected for mesenchymal stem cells (MSCs) and are good candidates for cell therapy applications and tissue bioengineering.
Keywords: Stem Cells; Pulp; Characterization; Differentiation.
RESUMO
Objetivo: Caracterizar as células-tronco pulpares e avaliar sua capacidade de expansão e diferenciação in vitro. Métodos: O tecido pulpar de terceiros molares permanentes foi retirado, digerido e as células plaqueadas em placas contendo meio HDMEM. Foi feita a expansão das células obtidas; foram realizados ensaios de diferenciação com meios indutores osteogênicos, condrogênicos e adipogênico; e caracterizado o perfil imunofenotípico com anticorpos específicos e avaliado por citometria de fl uxo. Resultados: Observou-se que as células-tronco pulpares apresentaram capacidade de aderência ao plástico e uma alta taxa de expansão e, após revelação com corantes específicos, se verificou que as células foram capazes de se diferenciarem em osteoblastos e condroblastos, porém não em adipócitos. A análise do fenótipo celular mostrou que as células foram negativas para CD45, CD69, CD117 e HLA-DR, e positivas para CD13, CD44, CD73, CD90 e CD105. Conclusão: As células isoladas de polpa dentária apresentaram características compatíveis com as esperadas para células tronco-mesenquimais (CTMs), sendo boas candidatas para trabalhos de terapia celular e bioengenharia de tecidos.
Palavras-chave: Células-Tronco; Polpa; Caracterização; Diferenciação.
INTRODUCTION
Stem cells are cells with a low degree of differentiation that have the capacity to reproduce and can generate differentiated cells of several different types of tissues1.
Tissue bioengineering has the capacity to accelerate regeneration and repair of damaged tissues using patients' natural curative potential and it is facilitated by several types of biomaterials2.
Many studies have demonstrated tissue bioengineering's potential and its applications for dentistry and regenerative medicine3-5. Pulp stem cells (PSCs) are able to form ectopic dentin in vitro and in vivo and also to generate a dentine-pulp complex composed of a mineralized matrix, with dentinal tubules that are aligned and filled with odontoblastic prolongations, containing vascularized pulp tissue, in a similar arrangement to that observed in natural dental structures3,6.
The objective of this study is to isolate and characterize stem cells from the pulp of permanent third molars and analyze the cells obtained in terms of their morphology and chondrogenic, osteogenic and adipogenic cell differentiation.
METHODOLOGY
This study was approved by the Human and Animal Research Ethics Committee at ULBRA (CEP-ULBRA 2009-361H) and all participants signed a free and informed consent form.
Ten healthy third molars were extracted at the orthodontist's recommendation and were immediately disinfected with 0.2% chlorhexidine digluconate followed by sectioning at the cementoenamel junction and removal of the pulp with a curette. The pulp tissue was then placed in a graduated tube containing Hepes buffered Dulbecco's modified eagle medium (HDMEM) (Figure 1) for transportation to the ULBRA Stem Cell and Gene Therapy Laboratory.
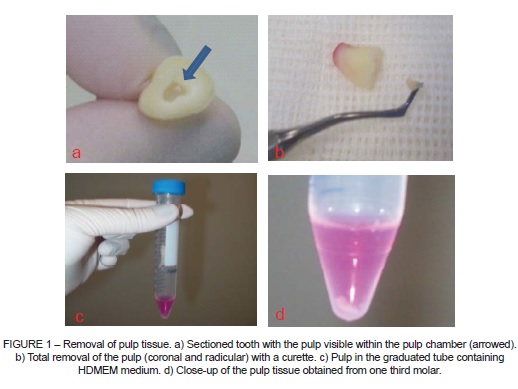
The pulp was later placed in the HDMEM medium, centrifuged and incubated at 37ºC. The cells were seeded onto six-well plates in DMEM medium containing 2.5 g/l of HEPES and supplemented with 10% fetal bovine serum, and the medium was changed after 72 hours to remove cells that had not adhered, while the cells that had adhered were transferred to a graduated fl ask for storage and culturing.
When the culture exhibited confl uence, the cells were passaged, using trypsin, expanded and counted in a Neubauer counting chamber, to monitor cellular kinetics.
During the fifth passage, the cultures were exposed to a series of cell differentiation inducers. Using a 12-well plate, three differentiation mediums were added to one threewell column each and one column was used to conduct a negative control with complete HDMEM medium only. The cultures were kept in the plate for 30 days, with medium changes every 4 days.
Osteogenic differentiation was induced using a culture medium containing complete HDMEM, Beta-glycerol phosphate, ascorbic acid (ASAp) and Dexamethasone. For chondrogenic differentiation, the cell cultures were placed in a medium containing complete HDMEM, insulin, TGF-Beta and ASAp. For adipogenic differentiation, Iscove's medium with dexamethasone, insulin, indomethacin and rosiglitazone was used.
After one month, the cultures were cured using alizarin for osteogenic differentiation and alcian blue for chondrogenic differentiation, while the adipogenic differentiation culture was stained with oil red. Each of the stains were used separately in the negative controls.
Finally, an immunophenotyping technique was used with the following specific antibodies: CD105, CD73, CD90, CD45, CD34, CD14/CD11b, CD79a/CD19, and HLA-DR.
RESULTS
Kinetics and cell morphology
Isolation of stem cells was only successful for four of the ten pulp samples collected. In these four cases it was observed that the cells had the capacity to adhere to the plastic of the culture flasks. PSCs have a similar morphological appearance to fibroblasts, with a polygonal shape and visible cytoplasmic prolongations (Figure 2).
On average, the stem cells remained in culture for 17 passages and maintained a high rate of expansion up to the most advanced passages, with a mean of 7.61x105 cells/ml.
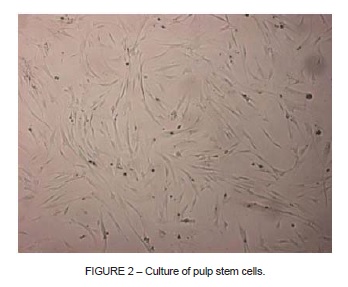
It was observed that during the initial passages, the mean time taken to achieve confl uence was 2 days, while the mean for the final passages was 4 days.
Cytometry and immunophenotyping
Analysis of the surface markers showed that the cells were negative for CD45, CD69, CD117 and HLA-DR, which are markers of hematopoietic lineages, and positive for CD13, CD44, CD73, CD90 and CD105, as can be observed in Figure 3.

Cell differentiation
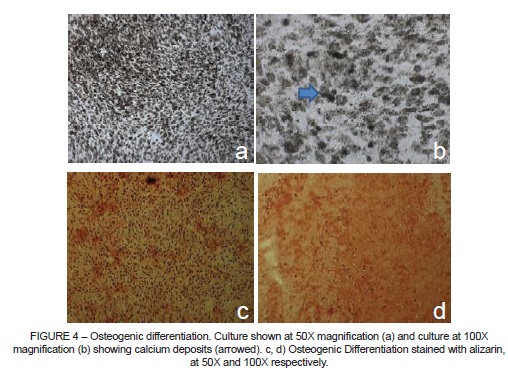
It was possible to observe PSC differentiation into osteoblasts in cultures kept in induction medium specific for bone differentiation (Figure 4). Staining with alizarin, which has an affinity for calcium, revealed deposition of an extracellular matrix rich in calcium over the culture.
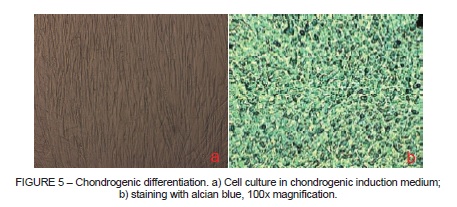
It was possible to observe PSC differentiation into chondroblasts in cultures kept in chondrogenic differentiation medium (Figure 5), which was confirmed by alcian blue staining, which stained them blue, demonstrating presence of chondroitin sulfate, one of the principal structural components of cartilage.
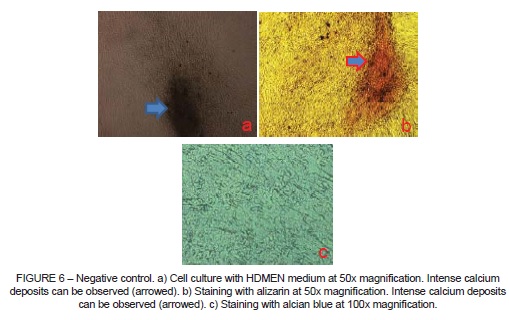
The negative control (Figure 6) was maintained in HDMEM culture medium only and in this case, even without osteogenic induction medium, it was possible to observe differentiation of the cells into osteoblasts. Cultures containing adipogenic induction medium did not differentiate.
DISCUSSION
There are several different protocols proposing methods for effective isolation of stem cells. The majority of them are based on the method proposed by Gronthos et al.3,7 and Gronthos et al.7, which is the method that was used in this study, with certain modifications. The protocol for isolation and culturing of the PSCs was very effective and the cells exhibited good proliferation results.
The stem cells exhibited an elongated and irregular shape with cytoplasmic prolongations (Figure 2), which are characteristics that can also be observed in fibroblasts. Although they are morphologically similar to fibroblasts, PSCs differ because fibroblasts do not have the capacity for self-renewal or transdifferentiation, whereas both of these properties are characteristic of the mesenchymal stem cells (MSCs) found in pulp. Other authors have also conducted studies in which cells morphologically similar to fibroblasts were observed8-10.
The immunophenotypical profile exhibited by the PSCs demonstrated that they were negative for CD45, CD69, CD117 and HLA-DR, which are markers of hematopoietic lineages, and positive for CD13, CD44, CD73, CD90 and CD105. These findings have also been described by several other authors3-11-13.
There is not yet consensus on a surface marker that is specific for stem cells, which is why a set of heterogeneous markers associated with the activity of undifferentiated mesenchymal cells is used14. According to the International Society for Cellular Therapy, it is consensus that identification of the markers CD105, CD73 and CD90 and absence of expression of hematopoietic markers is sufficient for immunophenotyping of these cells, as long as this characterization is combined with demonstration of cellular adhesion for long periods in culture and differentiation into at least two distinct cell lineages15,16.
It proved possible to induce the PSCs to differentiate into osteoblasts and chondroblasts, but not into adipocytes. In osteogenic differentiation treatments an intense deposition of calcium over the culture was observed (Figure 4), which was more easily seen when alizarin staining was applied6,17. Gronthos et al.3 described how after 6 weeks of osteogenic induction it was possible to observe nodules with high calcium content located sporadically throughout the adherent layer in the form of single mineralized zones that were positive for alizarin. The same result was observed in this study.
In chondrogenic differentiation treatments (Figure 5), formation of chondroblasts was observed and, by staining with alcian blue, formation of cartilage-like tissue with many collagen fibers could be observed, as described in many studies as type II collagen17-19.
The negative control, which was only cultured in HDMEM medium enriched with fetal bovine serum, spontaneously differentiated into bone matrix-producing cells (osteoblasts), even in the absence of osteogenic induction medium (Figure 6b), and when stained with alcian blue it was also observed that the negative control exhibited a slight tendency towards chondrogenic differentiation (Figure 6c), also spontaneous, but with a considerably weaker expression than its osteogenic differentiation.
CONCLUSIONS
The results observed allow for the conclusion that the cells isolated from the pulp of permanent third molars were compatible with MSCs, on the basis of the following principal characteristics: the capacity to adhere to plastic, a strong proliferative potential, clonogenic potential and their multi-lineage precursor potential, confirmed by osteogenic and chondrogenic differentiation, in addition to the phenotypical analysis results which showed that the PSCs were negative for hematopoietic markers, but positive for CD13, CD44, CD73, CD90 and CD105, which is a profile compatible with that expected of MSCs.
The possible applications for PSCs in odontology are highly diverse, since these PSCs can differentiate into several types of tissue. It was demonstrated that they can differentiate into odontoblasts, so they could be used to repair dental tissue lost to caries, and into osteoblasts, allowing patients to recuperate bone tissue, facilitating treatment of periodontitis and traumas, grafts for dental implants and treatment for bone pathologies. It was also shown that they are capable of chondrogenic differentiation, which could be an option for treatment of temporomandibular dysfunctions.
REFERENCES
1. Rao MS. Stem sense: a proposal for the classification of stem cells. Stem Cells Dev. 2004;13:452-5. [ Links ]
2. Tabata Y. Biomaterial technology for tissue engineering applications. J R Soc Interface. 2009;6:S311-24.
3. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625-30.
4. Dissanayaka WL, Zhu L, Hargreaves KM, Jin L, Zhang C. Scaffold-free prevascularized microtissue spheroids for pulp regeneration. J Dent Res. 2014;93:1296-303.
5. Gong T, Chin Heng B, Man Lo EC, Zhang C. Current advance and future prospects of tissue engineering approach to dentin/pulp regenerative therapy. Stem Cells Int. 2016;2016:9204574. doi: 10.1155/2016/9204574. Epub 2016 Mar 16.
6. Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S, et al. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res. 2003;12:976-81.
7. Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531-5.
8. Stanislawski L, Carreau JP, Pouchelet M, Chen ZH, Goldberg M. In vitro culture of human dental pulp cells: some aspects of cells emerging early from the explants. Clin Oral Invest. 1997;1:131-40.
9. Couble ML, Farges JC, Bleicher F, Perrat-Mabillon B, Boudeulle M, Magloire H. Odontoblast differentiation of human dental pulp cells in explants cultures. Calcif Tissue Int. 2000;66:129-38.
10. Huang GT, Sonoyama W, Chen J, Park SH. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res. 2006:324:225-36.
11. Suchanek J, Soukup T, Visek B, Ivancakova R, Kucerova L, Mokry J. Dental pulp stem cells and their characterization. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153:31-6.
12. Yalvac ME, Ramazanoglu M, Rizvanov AA, Sahin F, Bayrak OF, Salli U, et al. Isolation and characterization of stem cells derived from human third molar tooth germs of young adults: implications in neo-vascularization, osteo, adipo- and neurogenesis. Pharmacogenomics J. 2010;10:105-13.
13. Kang CM, Kim H, Song JS, Choi BJ, Kim SO, Jung HS, et al. Genetic comparison of stemness of human umbilical cord and dental pulp. Stem Cells Int. 2016;1:1-12.
14. Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofacial Res. 2005;8:191-9.
15. Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393-5.
16. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotentmesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-7.
17. Karbanová J, Soukup T, Suchánek J, Pytlík R, Corbeil D, Mokrý J. Characterization of dental pulp stem cells from impacted third molars cultured in low serum-containing medium. Cells Tissues Organs. 2011;193:344-65.
18. Mao JJ. Stem cells and the future of dental care. N Y State Dent J. 2008;74:20-4.
19. Eslaminejad MB, NazarianH, Shariati M, Vahabi S. Human dental pulp stem cells: the culture optimization for increased growth. Int J Hematol-Oncol Stem Cell Res. 2009;4:5-13.
 Correspondence:
Correspondence:
Alessandra Dutra da Silva
Curso de Odontologia
Rua Farroupilha, 8001
CEP 92425-900, Canoas
RS, Brazil
E-mail: dra.alessandradutra@gmail.com













