Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Stomatos
versão impressa ISSN 1519-4442
Stomatos vol.22 no.43 Canoas Jul./Dez. 2016
Oral manifestations in pediatric patients receiving chemotherapy for leukemia
Manifestações bucais em pacientes pediátricos submetidos a quimioterapia para leucemia
Milene Castilhos de Oliveira I; Tássia Silvana Borges II; Sergio Augusto Quevedo Miguens Jr. III Humberto Thomazi Gassen IV; Vania Regina Camargo Fontanella V
I Graduate Program in Dentistry, School of Dentistry, Universidade Luterana do Brasil (ULBRA), Av. Farroupilha, 8001, São José, prédio 59, 3º andar, Canoas/RS, Brazil
II DDS, MSc – Graduate Program in Dentistry, School of Dentistry, ULBRA, Av. Farroupilha, 8001, São José, prédio 59, 3º andar, Canoas/RS, Brazil
III DDS, MSc, PhD – Department of Oral Medicine, School of Dentistry, ULBRA, Av. Farroupilha, 8001, São José, prédio 59, 3º andar, Canoas/RS, Brazil
IV DDS, MSc – Department of Oral Medicine, School of Dentistry, ULBRA, Av. Farroupilha, 8001, São José, prédio 59, 3º andar, sala 21, Canoas/RS, Brazil
V DDS, MSc, PhD – Department of Oral Radiology, School of Dentistry, Federal University of Rio Grande do Sul, R. Ramiro Barcelos, 2492 – Santa Cecilia, Porto Alegre/RS, Brazil
The authors have no conflicts of interest to declare concerning the publication of this manuscript.
ABSTRACT
Cross-sectional study of 36 children with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML). Intraoral examination and oral hygiene assessment were performed in all patients. Information was also obtained from interviews of patients' mothers and a review of medical records. Results: The sample was largely composed of boys (58.3%), ages 5–9 years. The most commonly used chemotherapy regimen was the BFM-95 protocol. Most children (83.3%) had a record of some oral manifestation during treatment. On intraoral examination, 17 children (50%) were found to have at least one oral manifestation. The most frequent manifestation was also mucositis (26.5%), followed by gingival bleeding (23.4%). Lower maternal educational level was associated with increased frequency of mucositis, as were induction chemotherapy and maintenance chemotherapy after relapse. Conclusions: Oral abnormalities were common in children receiving chemotherapy and mucositis was the most prevalent manifestation. Oral lesions were associated with the induction phase of chemotherapy. Mucositis was not associated with oral health status.
Keywords: Leukemia; Chemotherapy; Oral Mucositis.
RESUMO
Estudo transversal de 36 crianças com leucemia linfoblástica aguda (LLA) ou leucemia mieloide aguda (LMA). Exame intraoral e avaliação de higiene bucal foram realizados em todos os pacientes. A informação também foi obtida a partir de entrevistas com as mães dos pacientes e uma revisão dos registros médicos. Resultados: A amostra foi composta em grande parte dos meninos (58,3%), com idades entre 5-9 anos. O regime de quimioterapia mais comumente utilizado foi o protocolo BFM-95. A maioria das crianças (83,3%) teve um registro de alguma manifestação oral durante o tratamento. Ao exame intraoral, 17 crianças (50%) apresentaram ter pelo menos uma manifestação oral. A manifestação mais frequente foi mucosite (26,5%), seguido por sangramento gengival (23,4%). Baixa escolaridade materna foi associada com aumento da frequência de mucosite, assim como a quimioterapia de indução e quimioterapia de manutenção após recaída. Conclusões: Anormalidades orais são comuns em crianças que receberam quimioterapia e mucosite foi a manifestação mais prevalente. As lesões foram associados com a fase de indução da quimioterapia. Mucosite não foi associado com o estado de saúde oral.
Palavras-chave: Leucemia; Quimioterapia; Mucosite Bucal.
INTRODUCTION
Childhood cancer corresponds to a group of diseases characterized by the uncontrolled proliferation of atypical cells, which may occur anywhere in the body1. The most common malignant neoplasms of childhood are the leukemias, central nervous system tumors, and the lymphomas2.
Among the leukemias, the most prevalent type is acute lymphoblastic leukemia (ALL), which is characterized by accumulation of immature lymphoid cells in the bone marrow2,3 and accounts for roughly 80% of all cases of leukemia in childhood. The age of peak incidence is 2–5 years, and approximately 60% of cases occur before the age of 20. The survival rate of children with ALL is nearly 90%4. Acute myeloid leukemia (AML), which accounts for 15–20% of all leukemias in childhood, belongs to a heterogeneous group of hematopoietic malignancies of monoclonal origin, which result from the malignant transformation of a stem cell. These neoplasms can manifest with a myeloid, erythroid, or platelet precursor phenotype, or a combination thereof5. The overall survival rate in children with AML is approximately 60–70%6.
Chemotherapy is the mainstay of treatment for all forms of leukemia. However, this treatment modality is necessarily associated with systemic or local adverse effects on healthy tissues. The oral manifestations associated whit chemotherapy are infl uenced by factors including the type, dosage, duration, and schedule of administration of chemotherapeutic agents; patient age; deficient oral hygiene; and preexisting periodontal disease7,8. These factors, in turn, influence the incidence of mucositis, decreased salivary flow (leading to xerostomia), pain (as a result of neurotoxicity), opportunistic infections (such as oral candidiasis), and gingival bleeding9.
Chemotherapy-associated oral mucositis occurs due to interference with epithelial cell turnover and induction of apoptosis10,11. The indirect cytotoxic effects of the release of infl ammatory mediators, loss of protective salivary components, and treatment-induced neutropenia have been noted as contributors to the development of oral mucositis12. Furthermore, chemotherapeutic agents such as fluorouracil, methotrexate, daunorubicin, and cyclophosphamide, which are commonly used in ALL treatment protocols, are particularly toxic to the oral mucosa13,14.
Dental follow-up should be considered during the various stages of leukemia treatment. Restoring oral health before antineoplastic treatment aims to prevent oral complications, whereas dental care during treatment seeks to manage these complications as they occur.
The objective of the present study was to evaluate oral manifestations in children receiving antineoplastic treatment for ALL or AML at a specialist pediatric cancer care center. We also sought to ascertain whether these complications are associated with oral hygiene status.
METHODS
Study design and setting
This cross-sectional study was conducted at the hematology/oncology clinic of Hospital da Criança Conceição (GHC), a children's hospital located in Porto Alegre, state of Rio Grande do Sul, Brazil, over a 12-month period.
Participants and eligibility criteria
The eligible population comprised all children with a diagnosis of leukemia who were undergoing chemotherapy at the time of the study, regardless of phase, and whose legal guardian provided written informed consent for participation in the study.
During the recruitment period, 38 patients met the inclusion criteria. Of these, two children participated in the pilot study and were subsequently excluded from analysis. Therefore, the final sample comprised 36 children with ALL and AML, aged 10 months to 15 years, of both sexes, who were being treated at GHC on an inpatient or outpatient basis.
Data collection
Data were collected from patient records, interviews with patients' mothers or guardians, and from the intraoral examination.
Paper-based and digital records from the GHC electronic medical records system were analyzed to collect demographic variables (sex, age, skin color, place of origin) and clinical parameters (comorbidities; type, phase, and protocol of antineoplastic therapy; dental evaluation before or during treatment).
Interviews were conducted using a semi-structured questionnaire designed to collect information on the educational level of the child's guardian and the child's oral hygiene habits, as well as to confirm clinical data obtained from medical records. The intraoral examination was performed in compliance with biosafety standards, under artificial lighting, with children properly positioned (seated or in the supine position), and always in the presence of a legal guardian. Two patients were lost to follow-up during this stage: one was discharged from the hospital and the other refused examination. Thus, 34 examinations were performed. Intraoral examination consisted of assessment of all oral soft tissues, including the lips, tongue, fl oor of mouth, cheek mucosa, palate, ridges/gums, buccal vestibule, and oropharynx. When present, mucositis was graded by severity (I to IV)15. Any opportunistic infections identified were classified by etiology as fungal, viral, or bacterial16,17. Patients were also examined for gingival hyperplasia and bleeding, mucosal pallor, and dry mucous membranes indicating decreased salivary flow. The extraoral examination was limited to evaluation of the presence of regional lymphadenopathy.
Oral hygiene status was evaluated by means of the Simplified Oral Hygiene Index (OHI-S), modified for the deciduous dentition when necessary18. Total OHI-S scores were classified as dental plaque absent (score = 0) or present (score > 1) and, subsequently, re-categorized as indicative of satisfactory or unsatisfactory oral hygiene, respectively19.
Data analysis
Data were analyzed in SPSS Version 20.0. Results were expressed as absolute and relative frequencies. The chi-square and Fisher's exact tests were used to evaluate associations of mucositis and oral hygiene with demographic and clinical variables. Significance was accepted at P < 5%.
Ethics
The study was approved by the Research Ethics Committee of Grupo Hospitalar Conceição – GHC (CEP/GHC: 116/08, FR: 202971). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
RESULTS
Of the 36 patients included in the study, 91.7% had a diagnosis of ALL with no associated conditions or comorbidities (83.3%). The sample was largely composed of boys (58.3%), ages 5–9 years (52.8%), from other municipalities (72.2%) (Table 1).
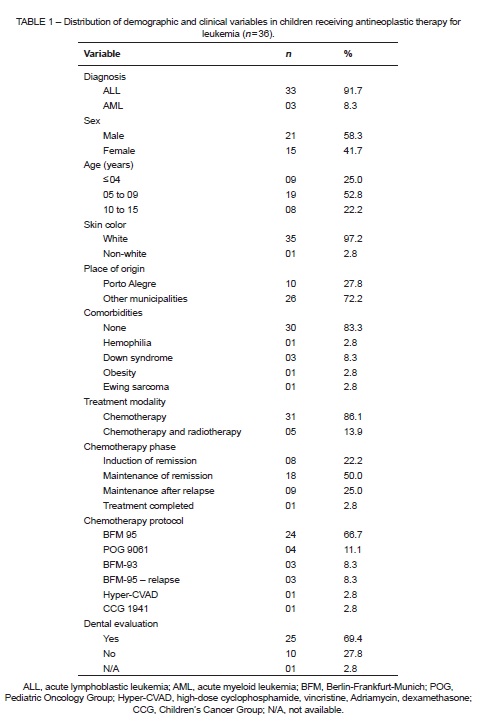
Regarding the treatment modalities employed, 31 children (86.1%) received chemotherapy alone, and only five (13.9%) received combined chemotherapy and radiation therapy. However, at the time of examination for the present study, all participants were undergoing chemotherapy exclusively. Regarding the phase of chemotherapy, 50% of children were in maintenance of remission, and the most commonly used chemotherapy regimen was the BFM 95 (Berlin-Frankfurt-Munich) protocol (66.7%). Twenty-five children (69.4%) had undergone dental evaluation by a hospital-affiliated dentist before or during treatment (Table 1).
Data obtained from charts showed that 30 children (83.3%) had a record of some oral manifestation, isolated or in combination, during treatment. The most prevalent manifestations were mucositis (73.3%), gingival bleeding (30%), herpesviral infections (26.7%), candidiasis (23.3%), and parotitis (10%).
On intraoral examination performed for the present study, 17 children (50%) were found to have at least one oral manifestation. The most frequent manifestation was mucositis (26.5%), followed by gingival bleeding (23.4%) (Table 2). No abnormalities were found on extraoral examination.
Most cases of mucositis identified were classified as grade I or II. There were no cases of grade III mucositis, and only one case of grade IV mucositis. The most commonly affected sites were the lip and tongue mucosa, alone or in combination with other areas (Table 3).
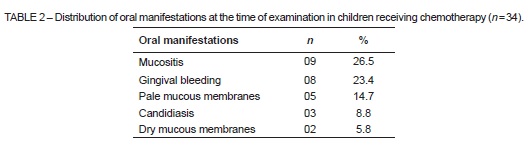
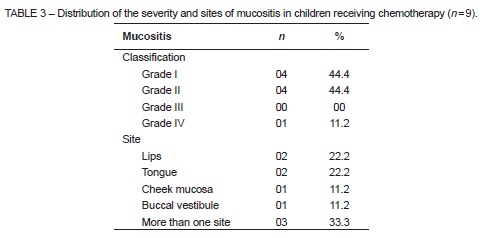
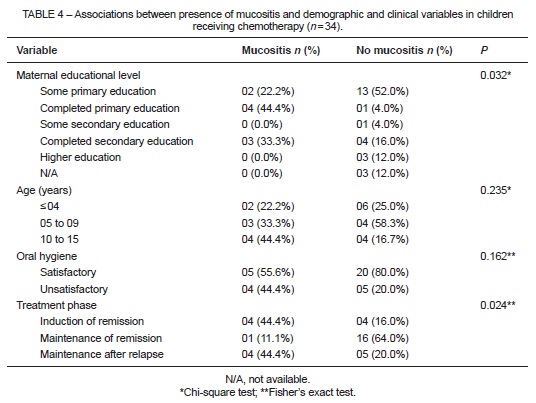
Table 4 shows the relationship between presence of mucositis and demographic and clinical variables. A significant association was found with maternal educational attainment (P = 0.032); children whose mothers had a lower educational level presented with a greater frequency of this complication. Phase of chemotherapy was also associated with mucositis (P = 0.024): the induction of remission and maintenance after relapse phases were those most often related. The highest percentage of children without mucositis was found among those receiving chemotherapy for maintenance of remission (64.0%).
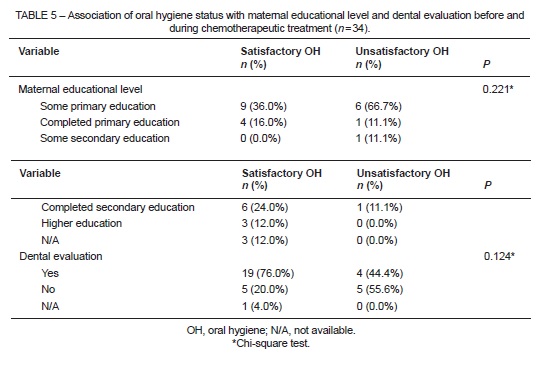
Oral hygiene status was considered satisfactory in 25 cases (73.5%), and was not associated with maternal educational level (P = 0.221) or with dental assessment by a hospital-affiliated practitioner (P = 0.124) (Table 5).
DISCUSSION
Over 80% of children participating in this study had a record of oral manifestations during chemotherapeutic treatment. At the time of intraoral examination, 50% of children had such complications, with a higher rate found among those under the age of 9. The younger the patient, the greater the likelihood of the oral mucosa being affected by chemotherapy; up to 90% of children under 12 experience oral complications7.
At the time of examination, all children were in chemotherapy, and mucositis was the most prevalent oral manifestation, regardless of chemotherapy phase or regimen. Oral mucositis is a complication generally associated with chemotherapy dosages or protocols that cause leukopenia secondary to neutropenia14,20. However, we were unable to test for an association of mucositis with neutropenia,17 as the patients included were at distinct treatment phases.
Investigation of the association between phase of chemotherapeutic treatment and presence of mucositis revealed a significant association (P = 0.024) with the remission induction and maintenance with recurrence phases, which were related to the highest frequencies of mucositis at the time of intraoral examination. This association may be attributable to the greater number of chemotherapy agents used in combination during these phases, such as vincristine, daunorubicin, and methotrexate, which have been implicated as the main causative agents of oral mucositis17. Of the patients analyzed, 66.7% were receiving the BFM 95 (Berlin-Frankfurt-Munich) protocol. This regimen involves drugs such as methotrexate, which is commonly associated with development of mucositis. On the other hand, the maintenance of remission phase of chemotherapy, which was the most frequent treatment stage at the time of examination, was also associated with the highest rate of children without mucositis (64.0%). This is consistent with the findings of Ribas and Araújo21. Episodes of mucositis most frequently affected the lip mucosa and lateral aspect of the tongue, which may be explained by the fact that these are areas of nonkeratinized mucosa22. Most lesions were classified as grade I or II. The frequency and severity of chemotherapy-induced mucositis may be related to risk factors inherent to each patient, such as type of leukemia, age, and oral hygiene status14,23,24.
Investigation of patient-related factors revealed an association of mucositis with maternal educational level (P = 0.032); children whose mothers had a lower degree of educational attainment exhibited higher rates of mucositis. However, it should be taken into consideration that all 18 children (50%) who exhibited satisfactory oral hygiene were in the maintenance phase of chemotherapy, which involves a protocol of fewer chemotherapeutic agents and, consequently, is associated with lower risk of oral manifestations.
Furthermore, considering that low household income25 and maternal educational level may infl uence the oral hygiene status of children,26 the mothers of 66.7% of the children who exhibited unsatisfactory oral hygiene had fewer than 8 years of formal education. However, no significant association was found (P = 0.221). The emotional status of the child and their family members regarding the disease and the treatment process itself should also be taken into account, as concerns with oral hygiene are relegated to the background in such situations.
On the other hand, the lack of awareness of the fact that oral hygiene habits play a relevant role in preserving or improving the overall health of the child reinforces the importance of including dentists in multidisciplinary oncology teams. Guidance, encouragement, and follow-up of oral health in these patients is paramount to preventing or mitigating the oral complications of antineoplastic therapy.
According to Hespanhol et al.,27 all patients who will receive chemotherapy must undergo prior dental examination to identify and treat potential foci of infection before development of granulocytopenia, and periodic monitoring should be performed throughout the course of treatment.
Gingival bleeding, observed in approximately 25% of cases in our sample, was also reported in previous studies28. This condition may be associated with thrombocytopenia secondary to myelosuppressive chemotherapy. Although the present study did not investigate this association, it is known that patients with systemic involvement who are receiving chemotherapy have deficiencies in coagulation and their gums may bleed at the lightest touch; this bleeding may be aggravated by unsatisfactory oral hygiene.
Other oral manifestations observed with a lower frequency in the present study included oral mucosal pallor, dry mucous membranes, and candidiasis. A previous study by Mendonça et al.29 also reported a lower frequency of these manifestations, which are believed to be associated with decreased salivary flow, neutropenia, and severity of mucositis. The mucosal dryness caused by chemotherapy usually represents a transient alteration in salivary gland function, and resolves shortly after conclusion of treatment or dose reduction28. This is consistent with the findings of the present study, in which only 8.8% of patients had candidiasis and 5.8% had dry mucous membranes at the time of examination; half of the sample was in the maintenance phase of chemotherapy, which involves a smaller amount of chemotherapeutic agents.
CONCLUSION
The ALL treatment protocols used at specialized cancer centers are often adapted. This practice should be taken into account when investigating oral conditions in their patients, who may present with oral manifestations of varying types and severities. Knowledge of the chemotherapy regimens, phases, and protocols used in the treatment of childhood leukemia is important for identifying associated oral manifestations, particularly mucositis. Constant evaluation and monitoring of oral hygiene and oral health is of the utmost importance in patients undergoing treatment for leukemia. Another important intervention is to educate, guide, and encourage patients, their family members, and the nursing team to maintain a satisfactory standard of oral hygiene, so as to prevent or minimize the risk of oral manifestations of antineoplastic treatment and improve patient quality of life.
REFERENCES
1. Curvo HRM, Pignatti WA, Pignatti MG. Morbidity and mortality from cancer children and adolescents associated with the agricultural use of pesticides in the state of Mato Grosso. Cad Saude Colet. 2013; 21 (1):10-17. [ Links ]
2. Brasil. Ministério da Saúde. Instituto Nacional de Câncer (INCA). Tipos de Câncer Infantil Accessed 2016 Jan 12. Available at: http://www2.inca.gov.br/wps/wcm/connect/ tiposdecancer/site/home/infantil.
3. Laks D, Longhi F, Wagner MB, Garcia PCR. Avaliação da sobrevida de crianças com leucemia linfocítica aguda tratadas com o protocolo Berlim-Frankfurt-Munique. J Pediatr. 2003;79 (2):149-158.
4. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943-1955.
5. Javed F, Utreja A, Bello Correa FO, Al-Askar M, Hudieb M, Qayyum F, Al-Rasheed A, Almas K, Al-Hezaimi K. Oral health status in children with acute lymphoblastic leukemia. Crit Rev Oncol Hematol. 2012;83 (3):303-309.
6. Chen SH, Jaing TH, Hung IJ, Yang CP, Chang TY. High body mass index did not result in poor outcome in Taiwanese children with acute myeloid leukemia: a single-institution experience. Int J Hematol. 2015;102 (1):48-52.
7. Martins ACM, Caçador NP, Gaeti WP. Complicações bucais da quimioterapia antineoplásica. Acta Scientiarum. 2002;24 (3):663-670.
8. Barasch A, Peterson DE. Risk factors for ulcerative oral mucositis in cancer patients: unanswered questions. Oral Oncol. 2003;39 (2):91-100.
9. Franch AM, Esteve CG, Pé rez MGS. Oral manifestations and dental management of patient with leukocyte alterations. J Clin Exp Dent. 2011;3 (1):53-59.
10. Blijlevens NM. Implications of treatment-induced mucosal barrier injury. Curr Opin Oncol. 2005;17 (6):605-610.
11. Lalla RV, Peterson DE. Oral mucositis. Dent Clin North Am. 2005; 49 (1):167-184.
12. Kostler WJ, Hejna M, Wenzel C, Zielinski CC. Oral mucositis complicating chemotherapy and/or radiotherapy: options for prevention and treatment. CA Cancer J Clin. 2001;51 (5):290-315.
13. Figliolia SL, Oliveira DT, Pereira MC, Lauris JR, Mauricio AR, Oliveira DT, Mello de Andrea ML. Oral mucositis in acute lymphoblastic leukaemia: analysis of 169 paediatric patients. Oral Dis. 2008;14 (8):761-766.
14. Chaveli-Lopez B. Oral toxicity produced by chemotherapy: A systematic review. J Clin Exp Dent. 2014;6 (1):81-90.
15. Santos PSS, Messaggi AC, Mantesso A, Magalhães MHCG. Oral mucositis: recent perspectives on prevention and treatment. RGO. 2009;57 (3):339-344.
16. Dholam KP, Gurav S, Dugad J, Banavli S. Correlation of oral health of children with acute leukemia during the induction phase. Indian J Med Paediatr Oncol. 2014;35 (1):36-39.
17. Morais EF, Lira JA, Macedo RA, Santos KS, Elias CT, Morais Mde L. Oral manifestations resulting from chemotherapy in children with acute lymphoblastic leukemia. Braz J Otorhinolaryngol. 2014;80 (1):78-85.
18. Greene JC, Vermillion JR. The Simplified Oral Hygiene Index. J Am Dent Assoc. 1964;68:7-13.
19. Cascaes AM, Peres KG, Peres MA, Demarco FF, Santos I, Matijasevich A, Barros AJ. Validity of 5-year-old children's oral hygiene pattern referred by mothers. Rev Saude Publica. 2011;45 (4):668-675.
20. Sonis ST. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol. 1998;34 (1):39-43.
21. Ribas MO, Araújo MR. Oral Manifestations in patients with leukemia. Rev Clin Pesq Odontol. 2004;12 (1):35-41. 22. Scully C, Sonis S, Diz PD. Oral mucositis. Oral Diseases. 2006;12 (3):229-241.
23. Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB, Hutchins RD, Peterson DE. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109 (5):820-831.
24. Mosel DD, Bauer RL, Lynch DP, Hwang ST. Oral complications in the treatment of cancer patients. Oral Dis. 2011;17 (6):550-559.
25. Feldens EG, Kramer PF, Feldens CA, Ferreira SH. Distribution of plaque and gingivitis and associated factors in 3- to 5-year-old Brazilian children. J Dent Child (Chic). 2006;73 (1):4-10.
26. Rahbari M, Gold J. Knowledge and behaviors regarding early childhood caries among low-income women in Florida: a pilot study. J Dent Hyg. 2015;89 (2):132-138.
27. Hespanhol LF, Tinoco EMB, Teixeira HGC, Falabella MEV, Assis NMSP. Buccal manifestations in patients submitted to chemotherapy. Cien Saude Col. 2010;15 (1):1085-1094.
28. Lopes IA, Nogueira DN, Lopes IA. Oral manifestations of chemotherapy in children from a cancer treatment center. Pesq Bras Odontoped Clin Integr. 2012;12 (1):113-119.
29. Mendonça RM, de Araujo M, Levy CE, Morari J, Silva RA, Yunes JA, Brandalise SR. Prospective evaluation of HSV, Candida spp., and oral bacteria on the severity of oral mucositis in pediatric acute lymphoblastic leukemia. Support Care Cancer. 2012;20 (5):1101-1107.
 Correspondence:
Correspondence:
Sergio Augusto Quevedo Miguens Jr.
Av. Farroupilha, 8001, São José
prédio 59, 3º andar
92425-900
Canoas/RS, Brazil
E-mail: samiguens@gmail.com













