Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Stomatos
versão impressa ISSN 1519-4442
Stomatos vol.23 no.44 Canoas Jan./Jun. 2017
Dental microscope as a useful tool to detect foramina in the furcation and pulp chamber floor of permanent teeth
Microscópio odontológico como uma ferramenta útil para detectar foraminas na furca e no assoalho da câmara pulpar de dentes permanentes
Leandro José Corrêa Harb I; Carine Weber Pires II; Fernanda Lavarda Ramos de Souza III; Tathiane Larissa Lenzi II; Maria Gabriela Pereira de Carvalho IV; Katia Olmedo Braun V; Carlos Alexandre Souza Bier IV
I Professor, Department of Morphology, Federal University of Santa Maria, Santa Maria, Brazil
II MSc, Graduate Program in Dental Science, Federal University of Santa Maria, Santa Maria, Brazil
III Dentist in Dental Clinic of Federal Institute Farroupilha, Jaguari, Brazil
IV Professor, Department of Stomathology, Federal University of Santa Maria, Santa Maria, Brazil
V Professor, Department of Restorative Dentistry, Federal University of Santa Maria, Santa Maria, Brazil
The authors have no conflicts of interest to declare concerning the publication of this manuscript.
ABSTRACT
This study assessed the influence of evaluation methods in the occurrence of foramina in the pulp chamber floor and in the furcation area of molars with complete and incomplete root formation. Methodology: A sample of 360 sound mandibular permanent molars was selected and prepared. A single experienced operator evaluated the whole sample using two methods: clinical inspection (with the naked eye) and dental microscope (at 30x magnification). Chisquare test was used to compare the detection of foramina between evaluation methods in both regions (p<0.05). Results: A limited number of specimens with foramina in the pulp chamber floor was observed, while there were more teeth with foramina in the furcation area, according both methods. The dental microscope identified significantly more molars with foramina in the furcation (p=0.000) and in the pulp chamber floor (p=0.031) than the clinical inspection. Conclusions: The presence of foramina in the furcation region is substantially greater than in the pulp chamber floor, regardless of the evaluation method. The presence of foramina is not influenced by the rhizogenesis stage. The dental microscope is an excellent tool to view dental anatomical details.
Keywords: Microscopy; Pulp Chamber; Anatomy; Molar.
RESUMO
Este estudo avaliou a influência dos métodos de avaliação na ocorrência de foraminas no assoalho da câmara pulpar e na área de furca dos molares com formação radicular completa e incompleta. Metodologia: Uma amostra de 360 molares permanentes inferiores hígidos foi selecionada e preparada. Um único operador experiente avaliou toda a amostra utilizando dois métodos: exame clínico (a olho nu) e microscópio odontológico (com aumento de 30x). O teste do qui-quadrado foi utilizado para comparar a detecção de foraminas entre os métodos de avaliação em ambas as regiões (p<0,05). Resultados: Observou-se um número limitado de espécimes com foraminas no assoalho da câmara pulpar, enquanto que havia mais dentes com foraminas na área de furca, de acordo com ambos os métodos. O microscópio odontológico identificou significativamente mais molares com foraminas na furca (p=0,000) e no assoalho da câmara pulpar (p=0,031) do que na inspeção clínica. Conclusões: A presença de foraminas na região de furca é substancialmente maior do que no assoalho da câmara pulpar, independentemente do método de avaliação. A presença de foraminas não é influenciada pelo estágio de rizogênese. O microscópio odontológico é uma excelente ferramenta para ver detalhes anatômicos dentários.
Palavras-chave: Microscópio; Câmara Pulpar; Anatomia; Molar.
INTRODUCTION
Internal dental anatomy consists of interconnected canals arranged in a complex root canal system. In this system, there may be accessory canals located in the interradicular region of permanent1-3 and primary molars2,4,5 that interconnect the pulp chamber and furcation area6-9. These canals, called foramina, are the result of a failure in the formation in Hertwig’s sheath during odontogenesis, probably due to blood vessels connected to the pulp, leading to an inadequate formation of dentin7.
Although accessory canals have been found throughout the root, those in the furcation area of molars are associated with more complications in the dental clinic4 due to their relation with dental pulp and periodontium. The main reasons for failure in endodontic treatment are usually the difficult access, effective cleaning and correct sealing10 Therefore, the contamination in these canals may induce small regions of pulp necrosis and calcifications and fatty degeneration in the pulp tissue may occur11. Furthermore, foramina in furcation are sites for biofilm deposits that turn very difficult for professional cleaning12 and may be the cause of furcation lesions13. Moreover, patients do have greater difficulty with hygiene, which explains the occurrence of failures in periodontal therapy with furcation involvement.
Since there are canals with different morphologies in the furcation region, the foramina do not necessarily show the presence of accessory canals in interradicular dentin. There are canals extended from the pulp chamber floor to the interradicular region of the tooth (real or type-A canals); canals that begin in the floor of the pulp chamber or in the furcation area, ending in interradicular dentin (blind or type-B canals); canals that begin in the floor of the pulp chamber or furcation area, crossing the interradicular dentin to end again in the pulp chamber floor or in furcation (loop or type-C canals); and confined canals in interradicular dentin, without communication (sealed or type-D canals)14.
Foramina in the pulp chamber floor may be observed by many methods including examination with the naked eye15 and the dental microscope16,17. The naked eye examination allows inference to the clinic, since it is the most used method in practice. To facilitate observation in laboratorial studies, some techniques can be performed, such as infiltration dyes by gravitational pressure, vacuum, soaking or centrifugation7,8,18,19. The dental microscope is commonly used in endodontics and it may contribute to the location of root canals13, especially those that could not be observed through the examination with the naked eye17.
The aim of this study was to evaluate the foramina in the pulp chamber floor and in the furcation area of mandibular permanent molars, using two different evaluation methods, as well as observing any differences in the occurrence of foramina in teeth with complete and incomplete root formation.
MATERIALS AND METHODS
Specimen preparation
This study was approved by the Ethics in Research Committee of the Federal University of Santa Maria (Registry number 0162.0.243.000-08).
A set of 360 mandibular permanent molars, with complete (260) and incomplete (100) rhizogenesis were randomly selected. The reasons for extracting these teeth were unknown. Teeth with intact pulp chamber floors and well exposed furcation regions (well separated roots in the root bulb region) were selected. Decayed teeth that presented change in form in the pulp chamber floor area, or in the cervical third of the crown, and teeth with fused roots or little exposed furcation were excluded. The teeth were rehydrated in distilled water at 37ºC for 14 days and the solution was changed every 48 h.
Two cuts were performed in each tooth using a high-speed diamond bur with constant refrigeration. The first cut was carried out to 1.5 mm apically to the furcation, and the second cut in the cement-enamel junction. The second cut surface was worn until 0.5 mm from the pulp floor. Then, root canals were expanded with #20 endodontic files associated with air/water syringes for pulp tissue removal.
The teeth were immersed in 1% sodium hypochlorite for 24 h in order to promote the solvency of adhered tissues to tooth surface20. Afterwards, they were washed in tap water and immersed again in the 1% sodium hypochlorite for ultrasound for 10 min. Finally, the teeth were washed in water and dried at room temperature to be individually stored.
Sample analysis
The teeth were immersed in trisodium EDTA (Biodinâmica, PR, Brazil) for 5 minutes, according to the manufacturer’s instructions, to improve the visualization of foramina. Images of the specimens with evident foramina in clinical inspection assessment were obtained, in a distance of 6 cm, with the macro function activated (Sony DSC-W5, Tokyo, Japan) (Figure 1). In the dental microscope assessment (Opto, Model DM 2003, Opto Eletrônica, SP, Brazil), the teeth images were taken by a digital camera (Nikon Coolpix 950, Tokyo, Japan) attached to the microscope (Figure 2). The teeth with complete and incomplete rhizogenesis were assessed only with the dental microscope.
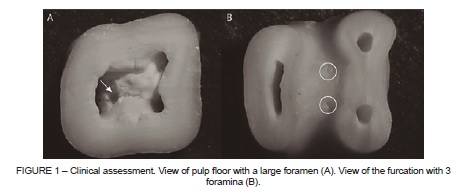
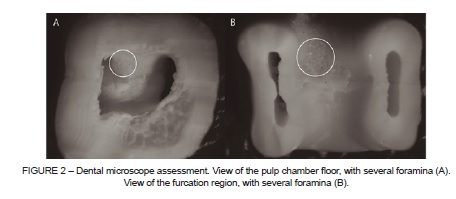
A single experienced operator was calibrated in both methods of evaluation: clinical inspection (with the naked eye) with aid of directional artificial light, to molars with complete (ICC=0.97 and incomplete (ICC=0.94) root formation; and dental microscope at 30x magnification, in teeth with complete (ICC=0.94) and incomplete (ICC=0.94) root formation. The whole sample was evaluated using the two methods by the same operator.
Statistical analysis
The data obtained of teeth with complete and incomplete rhizogenesis were descriptively analyzed. The Chi-square (χ2) test was used to compare the detection of foramina between the evaluation methods in both furcation and pulp chamber floor regions.
RESULTS
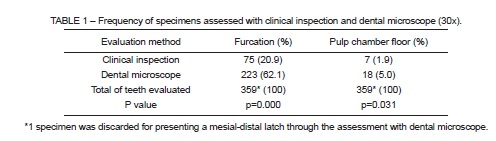
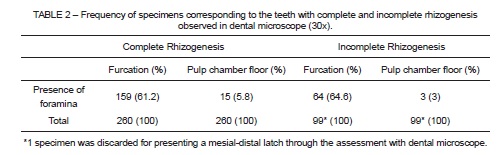
According to both evaluation methods, a limited number of specimens with foramina in the pulp chamber floor was observed, while there were more teeth with foramina in the furcation area (Table 1). The dental microscope identified significantly more molars with foramina in the furcation when compared to the clinical inspection (p=0.000). The same was observed when the pulp chamber floor was considered (p=0.031). There was a higher number of permanent molars presented foramina in the furcation region than in the pulp chamber floor, regardless of the rhizogenesis stage (Table 2).
DISCUSSION
The evaluation of presence of accessory canals between the pulp chamber floor and the furcation is relevant due to the association of these anatomic communications with endodontic and periodontal lesions. In this study, simple techniques, clinical inspection and dental microscope were used to locate accessory foramina, since they are closer to clinical reality considering the pulp chamber floor.
In the clinical inspection, only directional light on the specimen was employed, without the use of any instrument or endodontic file. This contributed to preserve the dental anatomy of the observed regions. According to this method, the majority of samples presented foramina in furcation area. This is in agreement with previous studies reporting frequencies that range from 8% to 64%1, 26% to 63%2, 25% to 92.5%3 in the pulp chamber floor and in the furcation region respectively, using scanning electron microscopy. Therefore, despite the clinical inspection being less accurate, it corresponds to results achieved with methods that are more precise.
Details of relief in both regions were viewed with the microscope, reaffirming its usefulness in accurately observing the dental anatomy. Results found when using a dental microscope corroborate previous studies that used a dissecting microscope. These reports found high prevalence of foramina in the furcation area (76%)12, and more teeth with lateral canals in the furcation (46%) than in the pulp chamber floor (13%)21. In the microscopic evaluation, significantly more teeth with foramina were observed than in the clinic evaluation. This contrast was expected to occur due to limitations of the natural evaluation method (human vision).
In the comparison of complete rhizogenesis and incomplete rhizogenesis groups, the percentages of teeth with foramina in both regions were similar. It does not match the expected result: although reparative or reactional dentin were not present in this sample, it was predicted that teeth with complete rhizogenesis would have fewer foramina22.
Considering the existence of various types of accessory canals14 and in accordance with previous studies3, the higher frequency of teeth with foramina in furcation compared to pulp chamber floor suggests that many foramina are not associated with the communications between the two surfaces (presence of real canals). Therefore, one can suggest that foramina are more related to periodontal lesions than to endodontic lesions, since when exposed to the oral environment, the furcation foramina can be bacterial deposits. This can make cleaning up the site difficult and jeopardize the success of periodontal treatment12. Despite the limitations of a laboratory study, the findings obtained in this study are useful to support the theoretical knowledge about therapeutic interventions.
CONCLUSIONS
The use of a dental microscope is an excellent tool to view dental anatomical details. The presence of foramina in the furcation is substantially greater than in the pulp chamber floor, regardless of the evaluation method. The number of foramina is not influenced by the rhizogenesis stage.
REFERENCES
1. Perlich MA, Reader A, Foreman DW. A scanning electron microscopic investigation of accessory foramens on the pulpal floor of human molars. J Endod. 1981; 7: 402-6. [ Links ]
2. Dammaschke T, Witt M, Ott KE, Schäfer E. Scanning electron microscopic investigation of incidence, location, and size of accessory foramina in primary and permanent molars. Quintessence Int. 2004; 35: 699-705.
3. Santana LNS, Freitas LB, Monteiro TL, Petta TM, Reis-Costa AC, Lima RR. Association between dentin thickness and presence of accessory foramina in human permanent mandibular molars. Braz J Oral Sci. 2011; 10: 233-5.
4. Ringelstein D, Seow WK. The prevalence of furcation foramina in primary molars. Pediatr Dent. 1989; 11: 198-201.
5. Kumar VDA. A scanning electron microscope study of prevalence of accessory canals on the pulpal floor of deciduous molars. J Indian Soc Pedod Prevent Dent. 2009; 27: 84-98.
6. Bender IB, Seltzer S. The effect of periodontal disease on the pulp. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1972; 33: 458-73.
7. Gutmann JL. Prevalence, location and patency of acessory canals in the furcation region of permanent molars. J Periodontol. 1978; 49: 21-6.
8. Maniglia CAG, Picoli F, Maniglia AB. Leakage study of the prevalence of furcation accessory canal in upper and lower human molars. Rev Fac Odontol Lins. 2004; 16: 41-6.
9. Quadros I, Zaia AA, Ferraz CCR, Souza Filho FJ, Gomes BPFA. Radiographic prevalence of root canal ramifications in a sample of root canal treatments in a Brazilian Dental School. Bras Oral Res. 2007; 21: 112-7.
10. Liu N, Li X, Liu N, Yr L, An J, Nie X, et al. A micro-computed tomography study of the root canal morphology of the mandibular first premolar in a population from southwestern China. Clin Oral Invest. 2013; 17: 999-1007.
11. Seltzer S, Bender IBE, Ziontz M. The Interrelationship of pulp and periodontal disease. Oral Surg Oral Med Oral Pathol. 1963; 16: 1474-90.
12. Burch JG, Hulen S. A study of the presence of accessory foramina and the topography of molar furcations. Oral Surg Oral Med Oral Pathol. 1974; 38: 451-5.
13. Vertucci FJ. Root canal morphology and its relationship to endodontic procedures. Endod Topics. 2005; 1: 3-29.
14. Paras LG. An investigation of accessory canals in furcation areas of human primary molars: Part 2. Latex perfusion studies of the internal and external furcation areas to demonstrate accessory canals. J Clin Pediatr Dent. 1993; 17: 71-7.
15. Vale IS, Bramante AS, Bramante CM. Presence of cavo-interradicular canal in upper and lower molars. Rev Odontol Univ São Paulo. 1996; 10: 207-14.
16. Carvalho MCC, Zuolo ML. Orifice locating with a microscope. J Endod. 2000; 26: 532-4.
17. Toubes KM, Côrtes MI, Valadares MA, Fonseca LC, Nunes E, Silveira FF. Comparative analysis of accessory mesial canal identification in mandibular first molars by using four different diagnostic methods. J Endod. 2012; 38: 436-44.
18. Niemann RW, Dickinson GL, Jackson CR, Wearden S, Skidmore AE. Dye ingress in molars: furcation to chamber floor. J Endod. 1993; 19: 293-6.
19. Haznedaroglu F, Ersev H, Odabaşi H, Yetkin G, Batur B, Aşçi S, et al. Incidence of patent furcal accessory canals in permanent molars of a Turkish population. Int Endod J. 2003; 36: 515-9.
20. Vertucci FJ, Anthony RL. A scanning electron microscopic investigation of accessory foramina in the furcation and pulp chamber floor of molar teeth. Oral Surg Oral Med Oral Pathol. 1986; 62: 319-26.
21. Vertucci FJ, Williams RG. Furcation canals in the human mandibular first molar. Oral Surg Oral Med Oral Pathol. 1974; 38: 308-14.
22. Katchburian E, Arana V. Oral histology and embriology, 2nd ed. Rio de Janeiro: Guanabara Koogan. 2004.
 Correspondence:
Correspondence:
Carine Weber Pires
Rua Floriano Peixoto, 1184 sala 211
Santa Maria, RS, Brazil
E-mail: cwpodonto@gmail.com













