Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.10 no.1 Piracicaba Jan./Mar. 2011
ORIGINAL ARTICLE
Antioxidant effect on the shear bond strength of composite to bleached bovine dentin
Masoomeh Hasani TabatabaeiI; Sakineh AramiI; Atefeh NojoumianI; Mansooreh MirzaeiI
I Department of Operative Dentistry, School of Dentistry, Tehran University/Medical Science, Tehran, Iran
ABSTRACT
Several studies have shown that compromised bonding to bleached enamel can be reversed with antioxidants. Aim: The aim of this study was investigate the effect of the antioxidant treatment on the micro-shear bond strength of a composite resin with a clinically acceptable antioxidant usage time taken into account. Methods: Using in vitro techniques, the effect of the antioxidant sodium ascorbate (SA) was evaluated on the micro-shear bond strength of a hybrid composite resin (Tetric® A2 Ivoclar Vivadent) to dentin, which was bleached with 35% carbamide peroxide (Opalescence Quick, Ultradent Products Inc). Thirty-five intact flat buccal dentin surfaces from bovine incisors were randomly assigned to five groups which were subjected to the following treatment protocols: group 1, bleached for 45 min and bonded immediately afterwards; groups 2 and 3, bleached and then treated with 10% SA for 10 and 5 min before bonding, respectively; group 4, stored in distilled water for seven days after bleaching and before bonding; group 5, received no bleaching or antioxidant treatment. After the bonding procedure, specimens were subjected to a micro-shear bonding test. Data were analyzed by ANOVA and a post-hoc Tukey's test. Results: One-way ANOVA revealed significant differences in bond strength among the five groups. Conclusions: It was found that the shear bond strength was reduced by carbamide peroxide bleaching, and that the antioxidant SA was ineffective at reversing the composite strength at the concentrations and treatment times examined.
Keywords: antioxidant, shear bond strength, composite, bleaching.
Introduction
Hydrogen peroxide bleaching is effective at lightening discolored teeth1. When bonding is performed immediately after bleaching, hydrogen peroxide and carbamide peroxide (CP) bleaching agents alter the bonding strength of composites to acid-etched enamel2-3. Delays in bonding of 1 to 3 weeks are recommended following bleaching, to avoid the clinical problems related to bleaching-mediated compromised bond strength4. One mechanism that may account for the lower bond strengths of bleached teeth is the presence of residual oxygen, which inhibits the polymerization of adhesive monomers5. Recent studies have revealed that the use of the antioxidant sodium ascorbate (SA) before the bonding process reverses the bleaching-induced reduction in bonding strength6-9. Antioxidants can neutralize free hydroxyl radicals and prevent their adverse biological effect10. Previous studies have different opinions about the duration of applying the antioxidant. The aim of this study was investigate the effect of this antioxidant treatment on the microshear bond strength of a composite resin with a clinically acceptable antioxidant usage time.
Material and methods
After slaughtering, 35 two-year-old intact bovine incisors were immediately extracted. The teeth were cleaned and sectioned at the cementoenamel level, pulp was removed using endodontic instruments, and the pulp chamber was rinsed with saline solution. The teeth were mounted with the buccal surface upwards in a self-curing acrylic resin using a heavy-body silicon mold. The teeth were stored in distilled water at 4oC for less than 3 months. Teeth were sectioned buccolingually parallel to the line axes at 2-mm slices, using a low speed machine (Isomet, Buehler Ltd., Lake Bluff, IL, USA) to expose the dentin. The dentin surface was ground (polished) with wet 600- and 1000-grit silicon carbide abrasive paper to create a flat dentin surface.
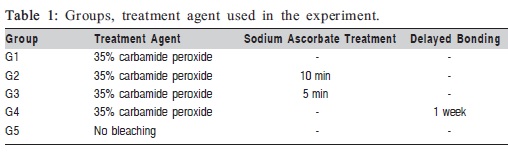
Specimens were randomly assigned to five groups (1 control and 4 experimental) of 7 teeth each (Table 1). Two or three composite samples were placed over each tooth, for a total of 17 specimens per group. The lingual sides of the teeth were placed in distilled water to simulate the humid condition of the oral cavity.
For the bleaching treatment, a 35% CP bleaching gel (Opalesence Quick, Ultradent Products Inc., USA) was placed on the dentin surfaces of the whole buccal aspect, and the teeth were placed in an incubator at 37oC and 100% humidity for 45 min. One group of teeth received no bleaching, as a positive control group (group 5). For the antioxidant treatment, a 10% SA solution was prepared by solving 10 g of SA powder (Merck KGaA, Darmstadt, Germany) in distilled water. SA solution was prepared immediately before its application on the teeth. Specimens from groups 2 and 3 were treated with 10% SA for 10 min and 5 min, respectively, followed by agitation with SA solution and thorough rinsing with tap water for 30 s. Specimens in group 4 were immersed in distilled water in an incubator at 37°C during 1 week after bleaching.
For the bonding treatment, two or three composite cylinders were applied to each sample. Application of the self-etching non-rinsing primer dentin adhesive system (Clearfil SE Bond, Kuraray Medical Inc., Japan) was according to the manufacturer's instructions. The above adhesive was used in this study because it is a current and clinically acceptable adhesive. The bonded surfaces were light-activated for 10 s using a visible light-curing unit (Optilux 501; Demetron Kerr, Danbury, CA, USA) with an output intensity level of 410 mW/cm2. Silicon tubes (0.7 mm internal diameter and 2 mm length) were placed on the dentin surface, and dental composite (Tetric Ceram, Ivoclar-Vivadent, Liechtenstein) was inserted into the tubes in one step. Each specimen was totally cured for 80 s. After curing, the silicon was removed and the specimens were subjected to 1000 thermal cycles between water baths of 5°C and 55oC, with a dwelling time of 15 s each. Specimens were then stored in distilled water at 4oC for one week.
The micro-shear bond strength was measured with a micro-shear testing machine (Bisco, Schaumburg, IL, USA) using a wire and loop method. The loop was prepared with orthodontic ligature wire, with one side placed around the sample and the other side around the testing machine rod. Specimens were loaded at a speed of 0.5 mm/min. Bond strengths (MPa) were calculated using the load required to debond the specimen.
Results were subjected to one-way ANOVA followed by a post-hoc Tukey's test at a 0.05% significance level. Statistical analysis was processed with SPSS for Windows XP. Differences with P < 0.05 were considered significantly different.
Results
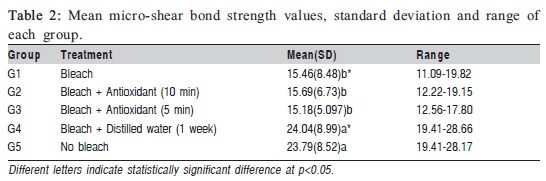
The results of the micro-shear bond strength tests are summarized in Table 2. One-way ANOVA revealed significant differences in bond strength among the five groups. The posthoc test indicated differences between groups 1 (CP + bonding), 2 (CP + SA for 10 min), 3 (CP + SA for 5 min) and groups 4, 5. The CP and two antioxidant groups (groups 2 and 3) showed significantly lower bond strength averages than the positive control (group 5) and the group where specimens were immersed in water for 1 week (group 4). No differences were observed when groups 2, 3, and 1 were compared with each other, and group 4 was not significantly different from group 5 (Table 2).
Discussion
In the present study, it was found that bleaching treatments of 35% CP decreased the micro-shear bonding strength of a hybrid composite resin to dentin. The use of 10% SA for 10 or 5 min before bonding was unable to neutralize the oxidizing effects of the bleaching agent.
Bond strength reduction by CP may be caused by the presence of residual peroxide, which can interfere with resin attachment and inhibit resin polymerization5,7-8. Previous scanning electron microscopy studies have shown that bleached resin interfaces display a few short, fragmented resin tags compared to interfaces with unbleached enamel. The bleaching of human tooth alters the enamel surface characteristics, causing surface loss and increased surface porosity2,9. These alterations may affect the bonding of composite resin, and contribute to microleakage if it is restored with a bonded composite11.
Bleaching can significantly reduce the relative concentrations of calcium and phosphorous and cause morphological alterations in the most superficial enamel crystallites12. Peroxide-containing bleaching agents affect the organic phase of enamel, affecting both the outer and inner enamel surfaces13. Theoretically, enamel pores, dentin and dentin fluid can all act as peroxide/oxygen reservoirs12,14. Dentin may be the most important reservoir of the three ones15, and is more affected by hydrogen or carbamide peroxide due to its lower mineral content and more organic matrix. Thus, dentin proteins may be denatured by hydrogen-based materials that produce morphological changes, which could negatively impact resin performances12,16. In particular, since the surface calcium levels affect dentin bonding13, it is predicted low shear bond strength values for dentin samples after bleaching.
Several methods have been proposed to avoid the clinical problems related to bleaching-associated reduced bond strength. The most common recommendation is delayed bonding for composite resin restorations16-18. Studies have identified 2 weeks as a satisfactory waiting period between bleaching and composite restoration for enamel and dentin17,19. Some in vitro studies indicate that the immersion of specimens in distilled water, saliva, or saline reverses the reduced enamel or dentin bond strength2-3,16. It is observed that the immersion of specimens in distilled water for 7 days after bleaching effectively reversed the reduced dentin bonding strength.
Enamel removal can also restore bonding strengths to their normal levels20. Barghi et al.1 reported that the adverse effects of enamel bleaching on bonding can be reduced or eliminated by treating the bleached surface with a water displacement solution, such as a dentin-bonding agent that contains acetone1. This has led others to recommend the use of alcohol-based bonding agents, particularly when restorative work is performed immediately after bleaching21.
The inorganic and organic components of both dentin and cementum are reportedly affected by 3% hydrogen peroxide22, which remains active in the pulp chamber or dentin tubules after bleaching due to its interaction with certain dentin components. Catalase has been suggested as an effective adjunct following bleaching to prevent the adverse effects of hydrogen peroxide10. Turkun et al.6 found that the bond strength of composite resin to bovine enamel increased after treating bleached teeth with 10% SA for 10 min6. Another report showed that antioxidant treatment for 10 min immediately after bleaching reversed the tensile bond strength of brackets23. Zhao et al.24 speculated that peroxide ions may be temporarily substituted for the hydroxyl radicals in the apatite lattice, producing a patite. It may be that these lattice substitutions are thermodynamically unfavorable, and may be reversed by an antioxidant9. On the other hand the use of SA to reverse bleaching-related oxidation required a substantial treatment time that may not be clinically acceptable9. Another study found that the use of a 10% SA hydrogel for 3 h before bonding neutralized the oxidizing effects of bleaching, increasing the enamel bond strength25. Also, Similar studies reported that compromised bonding can be reversed with 3 h application of hydrogel or solution from of sodium ascorbate26-27. Results of another study showed that the antioxidant gel should be applied to enamel for at least 60 min for maximum effectiveness28. Nevertheless, it is sought to reverse the bleaching-associated reduction in bond strength by neutralizing the residual oxygen with 10% SA, it is found that SA treatment before composite bonding was ineffective. Also, in an in vitro study to investigate the neutralizing effect of antioxidant agents on the bond strength of bleached enamel, Gomes Torres et al.15 found that only cataluse application increased the bond strength relative to the PC (positive control) group, and none of the tested treatments could completely neutralize the deleterious effects of bleaching. Although it could have been effective if the application time were increased to at least one-third of the bleaching time, this may not be clinically acceptable. Therefore, it is recommended that treatment be postponed for 1 week after bleaching.
The results of this study showed significant differences in bond strength among the five groups. The CP and two antioxidant group showed significant lower bond strength means than the control and distilled water-immersed (delayed bonding) groups. The effects of SA at different forms could be studied.
References
1. Barghi N, Godwin JM. Reducing the adverse effect of bleaching on composite-enamel bond. J Esthet Dent. 1994; 6: 157-61. [ Links ]
2. Nour EL-din AK, Miller BH, Griggs JA, Wakefield C. Immediate bonding to bleached enamel. Oper Dent. 2006; 31(1): 106-14.
3. Stokes AN, Hood JA, Dhariwal D, Patel K. Effect of peroxide bleaches on resin enamel bonds. Quintessence Int. 1992; 23: 769-71.
4. Cavalli V, Reis AF, Giannini M, Ambrosano GMB. The effect of elapsed time following bleaching on enamel bond strength of resin composite. Oper Dent. 2001; 26: 597-603.
5. Dishman MV, Covey DA, Baughan LW. The effects of peroxide bleaching on composite to enamel bond strength. Dent Mater. 1994; 9: 33-6.
6. Turkun M, Kaya AD. Effect of 10% sodium ascorbate on the shear bond strength of composite resin to bleached bovine enamel. J Oral Rehabil. 2004; 31: 1184-91.
7. Titely KC, Torneck CD, Ruse ND. The effect of carbamid-peroxide gel on the shear bond strength of a microfil resin to bovine enamel. J Dent Res. 1992; 71(1): 20-24.
8. Titely KC, Torneck CD, Ruse ND, Krmec D. Adhesion of a resin composite to bleached and unbleached human enamel. J Endod. 1993; 19: 112-5.
9. Lai SCN, Tay FR, Cheung GSP, Mak YF, Carvalho RM, Wei SH et al. Reversal of compromised bonding in bleached enamel. J Dent Res. 2002; 81: 477-81.
10. Rostein L. Role of catalase in elimination of residual hydrogen peroxide following tooth bleaching. J Endod. 1993; 19: 567-70.
11. Josey AL, Meyers IA, Romaniuk K, Symons AL. The effect of a vital bleaching technique on enamel surface morphology and the bonding of composite resin to enamel. J Oral Rehabil. 1996; 23: 244-50.
12. Perdigao J, Francci C. Ultra-morphological study of the interaction of dental adhesives with carbamid peroxide-bleached enamel. Am J Dent. 1998; 11: 291-301.
13. Hegedus C, Bistey T, Flora-Nagy E. An atomic force microscopy study on the effect of bleaching agents on enamel surface. J Dent. 1999; 7: 509-15.
14. Edward J, Swift JR, Perdigao J. Effects of bleaching on teeth and restorations. Compendium. 1998; 19: 815-20.
15. Gomes Torres CR, Fuzuko Koga A, Borges AB. The effects of antioxidant agents as neutralizers of bleaching agents on enamel bond strength. Braz J Oral Sci. 2006; 5: 971-6.
16. Basting RT, de Freitas PM, Pimenta LAF, Serra MC. Shear bond strength after dentin bleaching with 10% carbamide peroxide agents. Braz Oral Res. 2004; 18: 162-7.
17. Van der Vyver PJ, Lewis SB, Marais JT. The effect of bleaching agent on composite enamel bonding. J Dent Assoc S Afr. 1997; 52: 601-3.
18. Kayo AD, Tukun M. Reversal of dentin bonding to bleached teeth. Oper Dent. 2003; 6: 825-9.
19. Shinahara MS, Peris AR, Pimenta LA, Ambrosano GM. Shear bond strength evaluation of composite resin on enamel and dentin after non vital bleaching. J Esthet Restor Dent. 2005; 1: 22-29.
20. Cvitko E, Denehy GE, Swift EJ, Pires JA. Bond strength of composite resin to enamel bleached with carbamide peroxide. J Esthet Dent. 1991; 3: 100-2.
21. Sung EC, Chan SM, Mito R, Caputo AA. Effect of carbamide peroxide bleaching on the shear bond strength of composite to dental bonding agent enhanced enamel. J Prosthet Dent. 1999; 82: 595-9.
22. Rostein I, Lehr Z, Gedalia I. Effect of bleaching agents on organic components of human dentin and cementum. J Endod. 1992; 18: 290-3.
23. Bulut H, Kaya AD, Turkun M. Tensile bond strength of brackets after anti oxidant treatment on bleached teeth. Eur J Orthod. 2005; 27: 466-71.
24. Zhao H, Li X, Wang J, Qu S, Weng J, Zhang X. Characterization of peroxide ions in hydroxyapatitic lattice. J Biomed Mater Rest. 2000; 52: 157-63.
25. Kimyai S, Vafizadeh. The effect of hydrogel and solution of sodium ascorbate on bond strength in bleached enamel. Oper Dent. 2006; 4: 496-9.
26. Paul P, Rosaline H, Balagopel S. The effect of hydrogel and solution of sodium ascorbate on the bond strength of bleached enamel. J Conserv Dent. 2007; 10: 43-7.
27. Kimyai S, Savadi Oskoee S, Rafighi A, Valizadeh H. Comparison of the effect of hydrogel and solution forms of sodium ascorbate on orthodontic bracket-enamel shear bond strength immediately after bleaching: an in vitro study. Indian J Dent Res. 2010; 21; 54-8.
28. Kaya AD, Turkun M, Arici M. Reversal of compromised bonding in bleached enamel using antioxidant gel. Oper Dent. 2008; 33: 441-7.
 Correspondence:
Correspondence:
Sakineh Arami
Department of Operative Dentistry, School of
Dentistry, Tehran University/Medical Science,
Tehran, Iran
E-mail: nasrin.arami@gmail.com
Received for publication: August 13, 2010
Accepted: February 3, 2011













