Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.10 no.1 Piracicaba Jan./Mar. 2011
ORIGINAL ARTICLE
Influence of the respiratory mode and nonnutritive sucking habits in the palate dimensions
Luana Cristina BerwigI; Márlon Munhoz MontenegroII; Rodrigo Agne RitzelIII ;Ana Maria Toniolo da SilvaIV; Eliane Castilhos Rodrigues CorrêaV; Carolina Lisbôa MezzomoIV
I Speech-language pathologist, Master, Speech-language pathology Department, Federal University of Santa Maria, Santa Maria, Rio Grande do Sul, Brazil
II Dentist, Master student, College of Odontology, Federal University of Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil
III Otorhinolaryngologist, Master, Speech-language pathology Department, Federal University of Santa Maria, Santa Maria, Rio Grande do Sul, Brazil
IV Speech-language pathologist, PhD, Professor, Speech-language pathology Department, Federal University of Santa Maria, Santa Maria, Rio Grande do Sul, Brazil
V Physical Therapist, PhD, Professor, Physiotherapy Department, Federal University of Santa Maria, Santa Maria, Rio Grande do Sul, Brazil
ABSTRACT
Aim: To verify the influence of the respiratory mode and nonnutritive sucking habits in the transverse and vertical dimensions of the palate. Methods: Seventy-seven children aged 7 to 12 years, were divided, according the diagnosis of the respiratory mode and the presence of prolonged nonnutritive sucking habits. Models of the upper dental arc were obtained of all children for evaluation of the measures of the palate in the region of the canines, first and second premolars and first molars. These measures were analyzed by the Student's t-test and Analysis of Variance. Tukey's test was used for the multiple comparisons. The significance level was set at p<0.05. Results: It was verified that the mouth-breathing children showed smaller width and higher depth at the more posterior region of the palate. The children with prolonged nonnutritive sucking habits presented narrower and deeper palate at the anterior region of the palate. The canine distance was smaller in children who present mouth breathing associated to nonnutritive sucking habits and the depth at the second premolar was higher in mouth-breathers associated or not to prolonged nonnutritive sucking habits. Conclusions: The results suggest that the respiratory mode and prolonged nonnutritive sucking habits influence in the transverse and vertical palate dimensions in the children evaluated in this study.
Keywords: thumb-sucking, sucking behavior, palate hard, measures, mouth breathing child.
Introduction
The craniofacial growth and development were genetically determined. However, these processes can be affected by environmental factors such as sucking habits and mouth breathing, which change the muscular balance. This hypothesis is based on the functional matrix theory, according to which the soft parts acting on the different bone parts that compose the face would be the determinant factor of its growth and development1.
Based on the matrix functional concept, a previous study with rabbits in phase of facial growth and development demonstrated the importance of the facial musculature on the functional matrix for the determination of development of the skeletal facial of those rabbits2.
The nasorespiratory function and its relationship with the craniofacial growth has been a subject of great interest for over one hundred years. The modification of this function by a nasal airway obstruction or simply by mouth breathing habit can be, sometimes, associated to changes in the craniofacial complex3.
Depending on the frequency, intensity, duration and facial type, nonnutritive sucking habits also cause disturbances on the stomatognathic system due to the unbalance of the forces that naturally act in the oral cavity. These forces can favor the installation of the mouth breathing4-5.
Frequent adaptations of the orofacial complex commons to mouth breathing and prolonged nonnutritive sucking habits are: malocclusion, narrow maxilla, atresic palate, open lips at the rest position. tongue lowered on the floor of the mouth or protruded between the arches, flaccid orofacial musculature and atypical swallowing4,6-7.
The present study was carried out considering that the muscular and functional unbalance of the stomatognathic system due mouth breathing and pacifier and thumb sucking for a prolonged time could influence palate growth and development. The aim of this study was to verify the influence of the respiratory mode and nonnutritive sucking habits in the transverse and vertical dimensions of the palate.
Material and methods
The present study was approved by the Ethics in Research Committee of the Federal University of Santa Maria – CEP/UFSM protocol number 220.0.243.000-8.
A total of 273 children were screened from four public schools of Santa Maria city, Rio Grande do Sul state, Brazil. Children participation was possible after their parents/ guardians received full explanation about the research and expressed their agreement by signing a written consent form.
The exclusion criteria were: history of speech-language pathology and/or orthodontic treatment; evident signs of neurological impairment and/or syndromes; craniofacial malformations. The inclusion criteria were: to be 7 and 12 years old and Caucasian. From the 273 screened children, 77 were selected, 37 boys and 40 girls.
The selected children were subjected to the otorhinolaryngologic evaluation, which consisted of an interview with the children's parents and oroscopy, anterior rhinoscopy, otoscopy and nasofibrolaryngoscopy exams. From the diagnosis obtained in this evaluation, the children were classified as nose breathers (NB), when they breathed predominantly by the nose and mouth breathers (MB), when they breathed predominantly by the mouth.
The children were also classified according to the nonnutritive sucking habits by a Speech-language pathologist, who interviewed the parents and asked them about the presence and duration of pacifier and thumb sucking habits. These habits were considered prolonged when present for three years or more. Based on this information, the children were distributed in two groups: without habit (WHO) and with habit (WH).
The children were also grouped according to the respiratory mode and the nonnutritive sucking habits in:
- Nose breathing without habit (NBWOH) group
- Nose breathing with habit (NBWH) group
- Mouth breathing without habit (MBWOH) group
- Mouth breathing with habit (MBWH) group
Children were clinically examined by a dentist, who made alginate impressions and obtained cast models from the upper arch of each one of the 77 participants. Next, reference points were marked in the models for the transversal (width) and vertical (depth) measurements of the hard palate. The points were marked in the most apical palatal points of the maxillary canines, first and second premolars at the junction of the tooth and gingival margin8. In the first molars, the marked point corresponded to the union of the gingival margin with the palatal groove9 (Figure 1). In case of one tooth or both teeth had not erupted, these points were not marked and the measurements were not made in the respective level of the teeth.
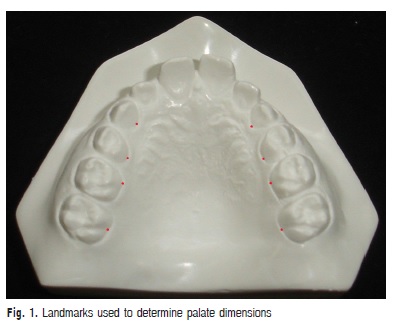
These measurements were made using a digital caliper (Western®) with 0.01mm of resolution and ± 0.02 mm of precision. For transversal measurements, the internal measuring faces of the instrument were used. For vertical measurements, a 0.05 mm stainless steel wire was cut with orthodontic pliers in the corresponding length to the transversal measurement and fixed with dental wax between the points in the level of each one of the considered tooth. After fixing the wire, the depth was measured with the depth measuring blade.
The following measurements of the palate were taken, obeying this order:
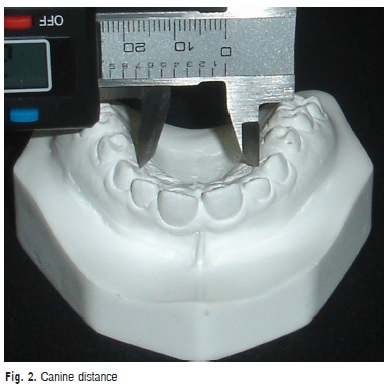
a) Canine distance: transversal distance in millimeters between the points of the gingival margin of the maxillary canine (Figure 2)
b) Canine depth: Vertical measurements in millimeters from the midpalatal line to the stainless wire that linked the gingival margin of the maxillary canine (Figure 3).
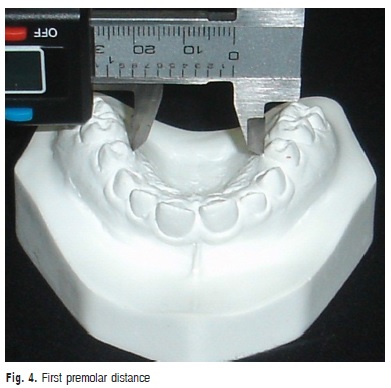
c) First premolar distance: transversal distance in millimeters between the points of the gingival margin of the maxillary first premolars (Figure 4).
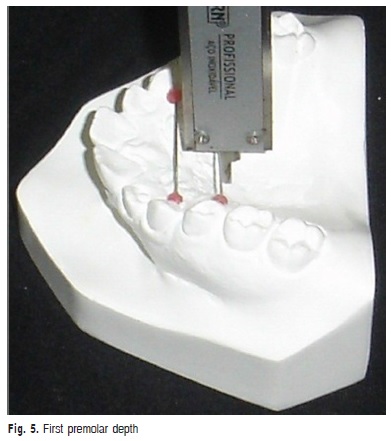
d) First premolar depth: vertical measurement in millimeters from the midpalatal line to the stainless wine that linked the gingival margin of the maxillary first premolars (Figure 5).

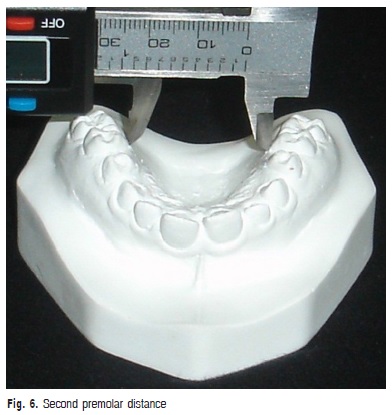
e) Second premolar distance: transversal distance in millimeters between the points of the gingival margin of the maxillary second premolars (Figure 6).
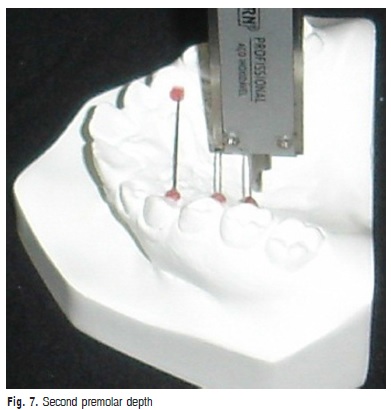
f) Second premolar depth: vertical measurement in millimeters from the midpalatal line to the stainless wire that linked the gingival margin of the maxillary second premolars (Figure 7).
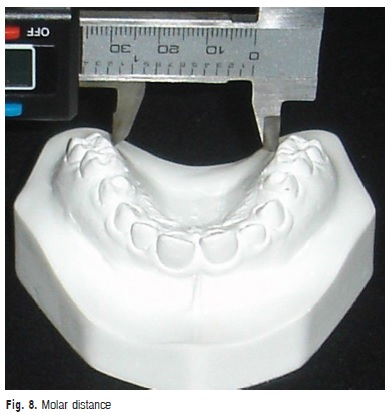
g) Molar distance: transversal distance in millimeters between the points of the gingival margin of maxillary first molars (Figure 8).
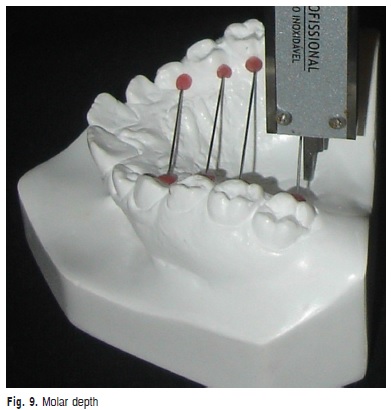
h) Molar depth: vertical measurement in millimeters from the palatine middle line to the stainless wire that linked the gingival margin of maxillary first molars (Figure 9).
After tabulation of the aforementioned measurements, the value of 0.05mm, corresponding to the diameter of the stainless wire was subtracted from the four depth measurements.
After 30 days, 30% of the models were randomly selected and reexamined by the same examiner to confirm the reproducibility of the palate measurements and verify the agreement between the first and second measurements by Intraclass Correlation Coefficient. It was verified significant agreement between all measurements. All variables presented normal distribution. The Student's t-test was used for the comparison between the palate dimensions between NB and MB, as well as between the WHO and WH groups. ANOVA and Tukey's multiple-comparison test were used for the comparison of the palate dimensions between NBWOH, NBWH, MBWOH and MBWH. A significant level of 5% was set for all analyses. Data were analyzed using the SPSS statistical software version 10.0 (2006).
Results
The means, standard deviations, minimal and maximal values of the measurements and the comparison of the palate dimensions among the studied groups are presented in Tables 1-3.
Table 1 shows statistically significant differences (p<0.05) in the mean values of the first and second premolars and molar distances and in the second premolar depth between the NB and MB groups. Table 2 shows statistically significant differences (p<0.05) in the mean of the canine distance and the canine and first premolar depth between the WOH and WH groups. Comparing the mean values of the palate dimensions among NBWOH, NBWH, MBWOH and MBWH (Table 3), statistically significant differences were observed in the canine distance and second premolar depth. Table 4 presents the results of the multiple comparisons. In the canine distance, there were statistically significant differences between the mean values of the NBWOH and MBWH. In the second premolar depth, there were statistically significant differences between the mean values of the NBWOH and MBWOH, as well as between NBWOH and MBWH.
Discussion
According to reviewed literature, few studies were found concerning the palate morphology or using a similar methodology to that of the present investigation. Therefore, the results of this study were compared to others with different methodologies of palatal measurements as: measurements in the mouth with three-dimensional Korkhaus compass10-11; with millimeter ruler12; measurements of cast models with three-dimensional Korkhaus compass13.
No previous study has investigated the relationship of between non-nutritive sucking habits and the palatal morphology, as analyzed by objective measurements. For this reason, the results of this study were compared to those of studies related to the dimensions of the upper dental arch.
The analysis of the palate dimensions, considering the respiratory mode, showed that all means of the transversal measurements were smaller and the mean of vertical measurements were larger in the mouth-breathing children than in the nose breathers. These differences were significant between the groups in the transversal distance in the region of the first premolars, second premolars and first molars and in the second premolar depth (Table 1).

These results suggests that there is a trend to palate narrowing in the posterior region in mouth breathers, as the mean of the transversal distance were significantly smaller at the first and second premolar and first molar regions in this group (Table 1). Likewise, Feres et al.13 (2009) verified significant difference between nose and mouth breathing groups in the measurements at the second premolar region.
Mouth-breathing children present narrower palate because the reduction of the air flow passage through the nasal cavity, which affect the lateral growth of the maxilla14. For this reason, the mouth-breathing patients frequently present posterior crossbite15-16.
The lack of significant difference between the groups in the mean of canine distance (Table 1) is in accordance with Feres et al.13 (2009),who compared mouth and nose breathers, and with Freitas et al.10 (2001) and Ghasempour, Mohammadzadeh and Garakani et al.11 (2009), who compared allergic and non-allergic patients. Based on these findings, it can be suggested that the respiratory mode is not related to the narrowing of the palate in its anterior region. Mouth-breathing children presented mean significantly higher in the depth of the palate at the second premolars region when compared to the nose breathers (Table 1). This result agrees with those of other studies that compared the palate depth in the region of these tooth between different respiratory modes and between children with and without allergic rhinitis10-13. The increase of the vertical dimension can occur due to the amplification of the pressure in the oral cavity in relation to the nasal cavity, which can justify the greater depth in the palate in the mouth-breathing group. However, the inverse relationship cannot be disposable, that is, patients with genetic tendency to a pattern of vertical facial growth and deep palate can develop the mouth breathing10-11.
The analysis of the palate dimensions according to the nonnutritive sucking habits revealed that WH children also presented all transversal dimensions smaller and vertical dimension larger than WHO children. However, this difference was significant, unlike the previous table, between the mean of the canine distance and canine depth and in the first premolar depth (Table 2). These results indicate that prolonged nonnutritive sucking habits are related with the narrowing and the greater depth in the anterior region of the palate, probably due to a higher pressure exerted in this region by the pacifier or the finger.
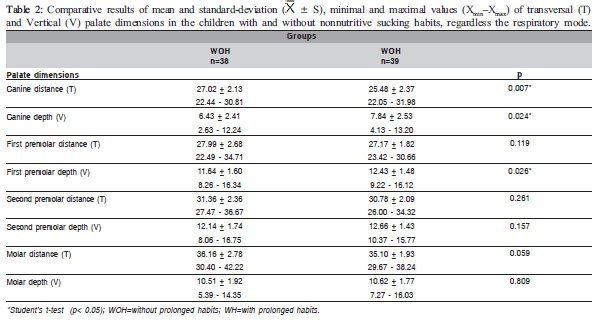
It has been reported that the prolonged nonnutritive sucking habits influence the dimension of the dental arches, with the decrease in the maxillary intercanine distance being frequently observed17-19.
Warren and Bishara20 (2002) verified that thumb and pacifier suction are associated with anterior open bite. Particularly, thumb sucking associated with increase of overjet and reduction in maxillary arch width and the pacifier to the increase of the mandibular arch width and with the posterior crossbite. In the present study, the palate dimensions were not analyzed according to the type of the nonnutritive sucking habits due to the low occurrence of the thumb sucking habit, present only in five participants of the study.
According to Larsson21 (1994), the prolonged use of pacifier is frequently associated with posterior crossbite due to the lowered posture of the tongue. This reduces the arch width and increases the risk of a transverse malrelationship between the upper and lower arches. The low tongue position widens the lower jaw in the same area thus enhancing the probability of the development of a posterior crossbite.
Comparing the mean of the palate dimensions between NBWOH, NBWH, MBWOH and MBWH (Table 3), there were statistically significant differences in the canine distance between NBWOH versus MBWH groups, as well as in the second premolar depth between NBWOH versus MBWOH and NBWOH versus MBWH (Table 4).
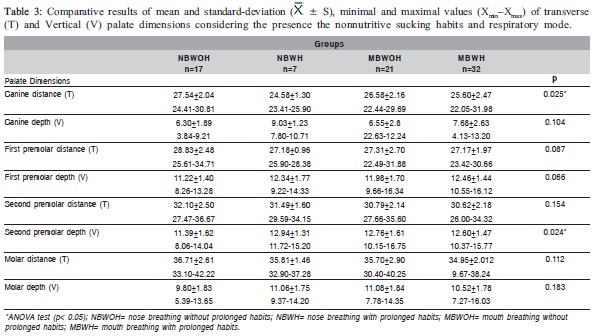
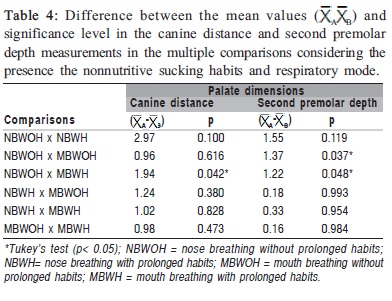
In order to compare the canine distance between NBWOH and MBWH (Table 3), the mean value was significantly smaller in the second group, suggesting that mouth breathing associated with prolonged nonnutritive sucking habits can cause narrowing of the anterior region of the palate.
The mean values of the second premolar depth were significantly greater in the MBWOH and MBWH compared to the NBWOH (Table 3). Again, these findings reveal that mouth breathing associated or not with the prolonged nonnutritive sucking habits can be related to the greater depth of the posterior region of the palate.
The increase of the palate depth and the decrease of the palate width, due to mouth breathing and the prolonged sucking habits can occur by the open lips at the rest position and the tongue lowered on the floor of the mouth or protruded between the arches. Under these conditions, there is not the external restraint by the lips and the tongue does not exert its function of expanding and modeling the palate9,22.
It can be concluded that the respiratory mode and the prolonged nonnutritive sucking habits had influence on the vertical and transverse dimensions of the palate in the children evaluated in the present study. When the respiratory mode was considered alone, it was observed smaller width and greater depth in the more posterior region of the palate. When the prolonged nonnutritive sucking habits were evaluated, it was observed a narrowing and greater depth of the anterior region of the palate. When the respiratory mode and the prolonged nonnutritive sucking habits were analyzed together, it was observed that the canine distance was smaller in the mouthbreathing children with nonnutritive sucking habits and the second premolar depth was greater in the mouth-breathing children with and without habits.
In view of the adverse manifestation in the craniofacial complex as a result of mouth breathing and prolonged nonnutritive sucking habits, a multidisciplinary team work is required. Parents should be oriented to search otolaryngologic treatment and to restrain the nonnutritive sucking habits as earlier as possible. Furthermore, orthodontic treatment is frequently needed for occlusal adjustments and adequacy of the facial musculature and the stomatognathic functions by means of myofunctional therapy. This way, the harmonic facial growth can be controlled and morphological changes can be reduced even in individuals with genetic predisposal.
Acknowledgements
Supported by "Coordenação de Aperfeiçoamento de Pessoal de Nível Superior" CAPES, Brazil.
References
1. Moss ML, Salentijn L. The primary role of functional matrices in facial growth. Am J Orthod. 1969; 55: 566-77. [ Links ]
2. Mateus AR, Dolci JE, Costa HO, Sousa FC, di Biase N. Experimental study on the influence of facial muscle activity on the facial mesostructure bones in rabbits. Braz J Otorhinolaryngol. 2008; 74: 685-90.
3. Vianna-Lara MS, Caria PH. Electromyographic analysis of the upper lip in nose and mouth breathers. Braz J Oral Sci. 2006; 5: 1203-8.
4. Degan VV, Puppin-Rontani RM. Removal of sucking habits and myofunctional therapy: establishing swallowing and tongue rest position. Pró Fono. 2005; 17: 375-82.
5. Almeida FL, Silva AM, Serpa EO. Relação entre má oclusão e hábitos orais em respiradores orais. Rev CEFAC. 2009; 11: 86-93.
6. Degan VV, Puppin-Rontani RM. Terapia miofuncional e hábitos orais infantis. Rev CEFAC. 2004; 6: 396-404.
7. Cattoni DM, Fernandes FD, Di Francesco RC, Latorre MR. Characteristics of the stomatognathic system of mouth breathing children: anthroposcopic approach. Pró Fono. 2007; 19: 347-52.
8. Laine T, Alvesalo L, Lammi S. Palatal dimensions in 45, X-females. J Craniofac Genet Dev Biol. 1985; 5: 239-46.
9. Oliveira MO, Vieira MM. Influência da respiração bucal sobre a profundidade do palato. Pró-Fono. 1999; 11: 13-20.
10. Freitas FC, Bastos EP, Primo LS, Freitas VL. Evaluation of the palate dimensions of patients with perennial allergic rhinitis. Int J Paediatr Dent. 2001; 11: 365-71.
11. Ghasempour M, Mohammadzadeh I, Garakani S. Palatal arch diameters of patients with allergic rhinitis. Iran J Allergy Asthma Immunol. 2008; 8: 63-4.
12. Perea PN, Quiñones JÁ, López AM. Determinácion de la profundidad del paladar em niños com respiración bucal de 6-8 años de edad. Rev Estomatol Hered. 2005; 15: 50-3.
13. Feres MF, Enoki C, Sobreira CR, Matsumoto MA. Dimensões do palato e características oclusais de crianças respiradoras nasais e bucais. Pesq Bras Odontoped Clin Integr. 2009; 9: 25-9.
14. Cappellette M, Carlini D, Pignatari SS, Cruz OL, Weckx LL. Rinometria acústica em crianças submetidas à disjunção maxilar. Rev Dent Press Ortod Ortop Facial. 2006; 11: 84-92.
15. Kiliç N, Oktay H. Effects of rapid maxillary expansion on nasal breathing and some naso-respiratory and breathing problems in growing children: A literature review. Int J Pediatr Otorhinolaryngol. 2008; 72: 1595-601.
16. Souki BQ, Pimenta GB, Souki MQ, Franco LP, Becker HM, Pinto JA. Prevalence of malocclusion among mouth breathing children: Do expectations meet reality?. Int J Pediatr Otorhinolaryngol. 2009; 73: 767- 73.
17. Warren JJ, Bishara SE, Steinbock KL, Yonezu T, Nowak AJ. Effects of oral habits' duration on dental characteristics in the primary dentition. J Am Dent Assoc. 2001; 132: 1685-93.
18. Zardetto CG, Rodrigues CR, Stefani FM. Effects of different paciûers on the primary dentition and oral myofunctional structures of preschool children. Pediatr Dent. 2002; 24: 552-60.
19. Aznar T, Galán AF, Marín I, Domínguez A. Dental arch diameters and relationships to oral habits. Angle Orthod. 2006; 76: 441-5.
20. Warren JJ, Bishara SE. Duration of nutritive and nonnutritive sucking behaviors and their effects on the dental arches in the primary dentition. Am J Orthod Dentofacial Orthop. 2002; 121: 347-56.
21. Larsson E. Artificial sucking habits: etiology, prevalence and effect on occlusion. Int J Orofacial Myology. 1994; 20: 10-21.
22. Moreira M, Lino AP. Evaluation of palatal depth and width in mouth breathers with primary dentition. Int J Orofacial Myology. 1989; 15: 19-24.
 Correspondence:
Correspondence:
Luana Cristina Berwig
Rua Tamanday, 533, CEP: 97060-540
Santa Maria/Rio Grande do Sul, Brasil
E-mail: luanaberwig@gmail.com
Received for publication: September 01, 2010
Accepted: February 03, 2011













