Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.10 no.1 Piracicaba Jan./Mar. 2011
ORIGINAL ARTICLE
Metabolic activity of Streptococcus mutans biofilms after treatment with different mouthwash formulations
Taciano R. CardosoI; Alexandre S. CarvalhoI; Marcelo E. BelettiII; Marcelo H. NapimogaIII; Geraldo Thedei JrI
I Laboratory of Biochemistry of Microorganisms, University of Uberaba, Uberaba, Brazil
II College of Veterinary Medicine, Federal University of Uberlandia, Brazil
III Laboratory of Biopathology and Molecular Biology, University of Uberaba, Brazil
ABSTRACT
Aim: The aim of this study was to investigate the metabolic activity of Streptococcus mutans biofilms after treatment with mouthwashes with different compositions. Methods: S. mutans biofilms were growth on polystyrene plates during 18 h, washed with sterile saline and treated with the following mouthwashes during 1 min: Listerine®, Oral B®, Parodontax® and Periogard® with and without alcohol. After the treatment, the biofilms were incubated with complete medium containing sucrose during 60, 120 or 180 min, and then samples were collected for pH measurements. In addition, biofilms were grown in microscope coverslips treated as described above, followed by staining with Propidium Iodide and Fluoresceine for visualization with a confocal laser scanning microscopy. Results: For all mouthwashes evaluated, treatment was deleterious to cell metabolism, since little or no acidification was observed at least 60 min after treatment. Mouthwashes containing 0.2% chlorhexidine (Parodontax®) or essential oils (Listerine®) induced a significant reduction in the metabolic activity of biofilms during the tested time points (120 and 180 min after treatment), being thus more effective than the mouthwashes containing 0.12% chlorhexidine (Periogard®) or cetylpyridinium plus fluoride (Oral B®). The confocal analysis overall confirmed the results observed in the analysis of metabolic activity. Conclusions: The treatment of biofilms with mouthwashes containing 0.2% chlorhexidine or essential oils induced significant reduction in S. mutans metabolism.
Keywords: Streptococcus mutans, mouthwashes, chlorhexidine, biofilm.
Introduction
Dental caries is a chronic contagious disease caused by several interacting factors, which results in the irreversible destruction of the mineralized structures of teeth, compromising their vitality and fixation in the maxillomandibular complex1,2.
The Gram positive bacteria Streptococcus mutans are a substantial part of the oral microbiota and their importance in the dental caries etiology is unquestionable3. The carbohydrates present in the diet are the main energy source in an anaerobic process (mainly lactic fermentation) resulting in the production of organic acids. These acids decrease the pH to around 4.5 on the tooth surface, thus inducing its demineralization4.
One important characteristic of S. mutans in promoting caries development is the ability to adhere firmly to the tooth surface in the presence of sucrose. This adherence is mediated mainly by the action of the GTF enzymes, which are considered fundamental to the virulence of S. mutans in the pathogenesis of dental caries 5-7.
Biofilm formation occurs as a result of a sequence of events: microbial surface attachment, cell proliferation, matrix production and detachment8. This process is partially controlled by quorum sensing, an interbacterial communication mechanism that is dependent on population density and is associated with radical changes in protein expression patterns8. Mature biofilms demonstrate a complex threedimensional structure with numerous microenvironments differing with respect to osmolarity, nutritional supply and cell density. Many antimicrobial agents that are effective against planktonic cells turn out to be ineffective against the same bacteria growing in a biofilm state9,10. Planktonic and biofilm cells also exhibit different susceptibilities to a certain antimicrobial concentration.
Several studies focusing on the efficacy of mouthwashes with diverse chemical composition demonstrated that combination of sodium fluoride and sodium lauryl sulfate as well as essential oils is able to diminish the metabolic activity of microorganisms present in the dental biofilm11-13.
Foster, et al.14 (2004) studied the effects of mouthwashes containing essential oils, triclosan, cetylpyridinium chloride and chlorhexidine against Streptococcus gordonii biofilms. The confocal laser scanning microscopy analysis demonstrated that all mouthwashes, except for cetylpyridinium chloride, were able to cause membrane damage after 60 s of incubation with S. gordonii biofilms.
Zhang, et al.15 (2004) evaluated the effect of a mouthwash with and without fluoride over metabolic activity of S. mutans biofilms and demonstrated that essential oil-containing mouthwashes, with or without 100 ppm of fluoride reduced the metabolic activity and the consequent acid production by approximately 36-44%. A significant reduction on total colony forming units (CFU) was observed in saliva of healthy volunteers after a single mouthwash with 0.2% or 0.12% chlorhexidine, but only the highest concentration showed bactericidal activity against salivary obligate anaerobes16. Furthermore, an in vivo study showed that both essential oils and alcohol-free chlorhexidine mouthwashes were able to reduce plaque acidogenicity after a sucrose challenge, with no difference between both solutions17.
Although several studies have been undertaken, little data are available about the action of mouthwashes with different active principles on bacterial biofilm metabolism, especially S. mutans biofilms, and the effects of those mouthwashes on three-dimensional structure of biofilms.
Material and methods
Mouthwashes
The following mouthwashes were evaluated in the present study: Parodontax® (Composition: 0.2% chlorhexidine gluconate (w/v), Batch: 168F, SmithKline Beecham Consumer Healthcare, United Kingdom); Listerine Cool Mint® (Composition: 0.092% eucalyptol (w/v), 0.042% menthol (w/v), 0.060% methyl salicylate (w/v), 0.064% thymol (w/ v), Batch: 3558B01, Johnson & Johnson, SP, Brazil); Oral- B® (Composition: water, glycerin, polysorbate 20, flavor, methylparaben, 0.053% monohydrated cetylpyridinium chloride, 0.050% sodium fluoride (226 ppm fluoride), sodium saccharine, sodium benzoate, propylparaben, ci 42090, ci 47005 batch: 8114852516, Rety Laboratories, Barranquilla, Colombia) and Periogard® with or without alcohol (Composition: 0.12% chlorhexidine gluconate (w/v), batch BR123A and BR112A, respectively, Colgate-Palmolive, São Bernardo do Campo, SP, Brazil). Positive and negative controls were 70% ethanol (v/ v) and sterile 0.9% (w/v) saline, respectively.
Streptococcus mutans growth conditions
The ATCC 25175 strain of S. mutans was purchased from the André Tosello Foundation, Campinas, SP, Brazil. The lineage was kept stored at -20ºC in 40% (v/v) glycerol (Sigma, St. Louis, MO, USA) medium and checked for purity before being grown in broth.
The frozen S. mutans cultures were reactivated in 5 mL of Triptic Soy Broth (TSB - Soybean-casein digest medium; Difco, Sparks, MD, USA) and incubated at 37°C, under microaerophylic conditions for 18 h. The cultures were adjusted to A620nm= 0.2 using a photocolorimeter (Analyser Com & Ind. LTDA. São Paulo, SP, Brazil) and 750 mL of this suspension was transferred to a tube containing 30 mL of previously autoclaved complete medium18 (10 g/L tryptone, 5 g/L yeast extract, 60 μmol/L MgSO4, 1.3 μmol/L FeSO4, 1.5 μmol/L MnCl2, 0.2 mmol/L KH2PO4, 0.3 mmol/L K2HPO4, 0.7 mmol/L KCl, pH 7.0) supplemented with 50 mMol/L sucrose as carbon source. Then, 600 mL of this suspension was inoculated in a 24-well cell culture plate (Corning Costar 3524, flat bottom) and incubated at 37°C, under microaerophilia, during 18 h. for biofilm formation as previously described7.
Effects of mouthwashes on S. mutans metabolism
All procedures were carried out in as a blind experiment. After biofilm formation as described above, the culture medium of each well was removed and the pH was measured using a PG 1800 pH meter associated with a microelectrode (Gehaka, São Paulo, SP, Brazil). The formed biofilms were washed 3 times with sterile 0.9% (w/v) saline and 1 mL of each the mouthwashes was added to each well. After 1 min of incubation, the mouthwashes were removed and the wells washed with abundant sterile 0.9% (w/v) saline. Then, to each well was added 1 mL sterile complete medium supplied with 50 mMol/L sucrose as carbon source. The treated biofilm was incubated at 37ºC under microaerophilic conditions and samples were taken at 60, 120 and 180 min for further pH analysis.
The positive control used was ethanol 70% (v/v) and the negative control was sterile 0.9% (w/v) saline.
Confocal Laser Scanning Microscopy (CLSM)
For the CLSM study, glass coverslips were inserted in previously autoclaved Falcon Tubes with 30 mL of complete medium18 supplemented with 50 mMol/L sucrose as carbon source. Suspension of 5 x 107 bacteria/mL of S. mutans were added and cultivated for 18h. The S. mutans biofilm formed in the coverslips were washed and treated with different mouthwashes during 1 min. After that, the coverslips were extensively washed with sterile saline and treated with 1 mM propidium iodide followed by 0.1% fluoresceine. The coverslips were mounted on individual slides and the images was captured for an emission wavelength at 500-530 nm or at 600-675 nm respectively at 63× magnification with a confocal laser scanning microscope (Carl Zeiss LSM 510 META, Jena, Germany). The two color images obtained by a CLSM, i.e. a green-filtered emission image and a red-filtered emission image, were converted to digital image and merged together using the Zeiss LSM Image Browser.
Statistical analysis
Data are reported as the mean of triplicate measurement of three independent assays. One-way analysis of variance (ANOVA) was used to determine the significance between treatments. To determine whether the means were statistically different from each other we used the Bonferroni's multiple comparison test, considered to be statistically significant at P<0.05 or less.
Results
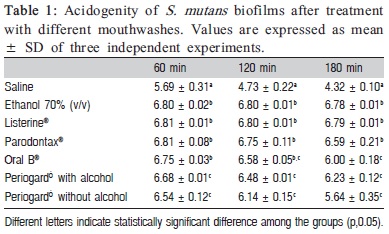
After 60 min of the mouthwashes treatment, all of the tested brands of mouthwashes differ significantly (p<0.05) from saline-treated biofilm (control), avoiding a more intense decrease in the pH. However, Periogard® with or without alcohol demonstrated a more intense pH-drop, differing significantly (p<0.05) from the other mouthwashes evaluated (Table 1, column 1), suggesting lower efficacy. In the second time analyzed (120 min after the treatments), the saline-treated biofilm showed an intense pH-drop statistically significant (p<0.05) when compared to all mouthwashes. Moreover, Ethanol 70, Listerine®, Parodontax® and Oral B® demonstrated a slight pH-drop avoiding high variations and none of them differed statically among them (p>0.05). However, the biofilm treated with Periogard® with or without alcohol demonstrated a higher pH-drop (acidification) statistically significant (Table 1, column 2). Finally, when analyzing the biofilm acidification 180 min after mouthwash treatment, saline-treated biofilm had a very low pH, characteristically demonstrating the metabolic activity and viability of the biofilm. Furthermore, Ethanol 70, Listerine® and Parodontax® showed the best effect avoiding an intense pH-drop, while Oral B®, Periogard® with or without alcohol showed the lowest pH, differing significantly (p<0.05) from all other mouthwashes (Table 1, column 3).
CLSM was used to ascertain the viability of bacteria in the biofilm after mouthwash treatment. S. mutans biofilm without any treatment revealed great cell viability (Fig. 1A), contrasting with a higher level of dead cells after 70% ethanol treatment (Fig. 1B). Furthermore, biofilm treated with mouthwashes containing essential oils (Listerine®) or 0.2% chlorhexidine (Parodontax®) caused extensive damage to biofilms (Fig. 1C and D, respectively), comparable to or more extensive than lesions induced by ethanol. It was also possible to observe that both antimicrobial agents used effectively penetrated the biofilm. In a smaller extent, treatment of biofilms with 0.12% chlorhexidine plus alcohol (Periogard® with alcohol, Fig. 1E) also was able to cause cell death, whereas alcohol-free 0.12% chlorhexidine (Periogard® without alcohol, Fig. 1F) and alcohol-free cetylpyridinium chloride plus fluoride mouthrinse (Oral B®, Fig. 1G) caused a low level of cell death, restricted to spots on biofilm and not throughout the biofilm. These results, in a greater extent, are corroborative with pH measurements after treatment of biofilms with mouthwashes (Table 1).
Discussion
The formation of dental biofilm is instantly initiated after tooth cleaning by the adsorption of salivary components to the enamel surface, followed by addition of initial colonizers, to which eventually, the climax community of matured dental biofilm will adhere11,19. Biofilm bacteria are involved in a matrix of salivary proteins and microbial products20. This type of growth protects the bacteria from external agents, such as antibiotics11, and mouthwash components21.
In the present study, the mouthwashes with essential oils and 0.2% chlorhexidine showed similar efficacy to 70% (v/v) ethanol to reduce the acidogenicity from S. mutans biofilms (Table 1). These results are in agreement with those of a recent study17, which demonstrated in vivo that using mouthwashes with essential oils or alcohol-free chlorhexidine during a 16-day period reduced plaque acidogenicity after a sucrose challenge.
Kocak, et al.22 (2009) showed that 0.12% chlorhexidine was effective against oral microorganisms. Our results suggest that an alcohol-free mouthwash containing 0.12% chlorhexidine was able to reduce the bacterial metabolism as compared to the negative control, but failed, at any time evaluated, to reduce significantly the bacterial metabolism as compared to the positive control. The in vivo study of those authors22 evaluated the efficacy of mouthwashes measuring the number of S. mutans CFU present in saliva after use of mouthwash, probably reflecting only cells that detached from biofilm and not the whole dental biofilm. In the present study, the whole biofilm was analyzed and the results clearly showed that 0.12% chlorhexidine failed to eliminate the metabolic activity and also to induce extensive membrane damage to biofilm growing S. mutans. Thus, this result indicates that chlorhexidine concentration is determinant to its penetrability into the biofilm. Tomás, et al.16 (2008) observed a reduction of total bacterial population after use of both 0.2% and 0.12% chlorhexidine mouthwashes. However, these authors16 also reported that only the higher concentration showed bactericidal activity, which agrees with our results for both acidogenicity and CLSM assays.
Comparison between 0.12% chlorhexidine with and without alcohol showed a small advantage of the alcoholcontaining mouthwash, since it caused a 60 min delay in acidogenicity in comparison to the alcohol-free version (Table 1). A similar result was found in a previous study23 that compared two chlorhexidine solutions against plaque re-growth and bacterial viability, showing that ethanol may contribute significantly to reduce bacterial vitality. Interestingly, in the present study the worst results were obtained from alcohol-free mouthwashes, suggesting that the alcohol may contribute to a better penetrability of the active principle into the biofilm.
Witt, et al.24 (2005) observed no difference between an alcohol-free cetylpyridinium chloride mouthwash and a product containing essential oils, when using a Modified Quigley-Hein Plaque Index. On the other hand, in the present study, the cetylpyridinium chloride plus fluoride mouthwash had the worst capacity to reduce S. mutans metabolism (Figure 1), as shown in both acidogenity and CLSM experiments. Among the reasons to explain these results, we can arise that: (1) the penetrability of cetylpyridinium chloride might not have been sufficient to entirely permeate the biofilms; (2) the molecule could penetrate but the contact period between cetylpyridinium chloride and bacterial cells was insufficient to cause membrane damage; or (3) the cetylpyridinium chloride concentration present in the mouthwash used was below of the necessary to cause extensive membrane damage.
Our data from CSLM strongly suggests that reduction of metabolic activity is due to cell damage as a result of mouthwash treatment. In the present study, among 5 mouthwashes tested, only 2 showed efficient penetration of the agents throughout the biofilm as observed in the positive control experiment, visualized by CLSM. Evidence of membrane damage extended from the bottom of coverslips to the surface of biofilms induced by 0.2% chlorhexidineand essential oil-containing mouthwashes suggests an effective penetration of these molecules through the biofilm. Interestingly, 0.12% chlorhexidine showed poor efficacy when compared to 0.2% chlorhexidine, indicating that a small variation in concentration may compromise the penetrability and, consequently, bacterial inactivation.
Several previous studies17,22,23 measured the efficacy of antimicrobials on dental plaque in vivo and some of these studies had high interindividual variations of the results17. The methodology employed in the present study is highly reproducible, low cost and easy to perform. Furthermore, it was attempted to mimic exposure times often used in in vivo clinical studies (60 s)25-27. Thus, it may be concluded that the mouthwashes containing essential oils or 0.2% chlorhexidine showed higher efficacy than those containing cetylpyridinium chloride plus fluoride or 0.12% chlorhexidine.
Acknowledgements
The authors would like to thank UNIUBE and FAPEMIG for the continuous support given to our laboratories, and Hilara N. Ruas for technical assistance. TRC and ASC were recipients, respectively, of Master's degree and undergraduate fellowships from FAPEMIG.
References
1. Krasse B. Caries risk: A pratical guide for a assessment and control. Chicago: Quintessence; 1985. p.113. [ Links ]
2. Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003; 149: 279-94.
3. Mikkelsen L, Jensen SB, Jakobsen J. Microbial studies on plaque from carious and caries-free proximal tooth surfaces in a population with high caries experience. Caries Res. 1981; 15: 428-35.
4. Chestnutt IG, MacFarlane TW, Aitchison TC, Stephen KW. Evaluation of the in vitro cariogenic potential of Streptococcus mutans strains isolated from 12-year-old children with differing caries experience. Caries Res. 1995; 29: 455-60.
5. Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986; 50: 353-80.
6. Kuramitsu HK. Virulence factors of mutans streptococci: role of molecular genetics. Crit Rev Oral Biol Med. 1993; 4: 159-76.
7. Mattos-Graner RO, Napimoga MH, Fukushima K, Duncan MJ, Smith DJ. Comparative analysis of Gtf isozyme production and diversity in isolates of Streptococcus mutans with different biofilm growth phenotypes. J Clin Microbiol. 2004; 42: 4586-92.
8. Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol. 2002; 184: 1140-54.
9. Drenkard E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect. 2003; 5: 1213-9.
10. Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005; 13: 34-40.
11. Petersen FC, Assev S, Scheie AA. Combined effects of NaF and SLS on acid- and polysaccharide-formation of biofilm and planktonic cells. Arch Oral Biol. 2006; 51: 665-71.
12. Filoche SK, Soma K, Sissons CH. Antimicrobial effects of essential oils in combination with chlorhexidine digluconate. Oral Microbiol Immunol. 2005; 20: 221-5.
13. Takarada K, Kimizuka R, Takahashi N, Honma K, Okuda K, Kato T. A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol Immunol. 2004; 19: 61-4.
14. Foster JS, Pan PC, Kolenbrander PE. Effects of antimicrobial agents on oral biofilms in a saliva-coated flowcell. Biofilms. 2004; 1: 3-10.
15. Zhang JZ, Harper DS, Vogel GL, Schumacher G. Effect of an essential oil mouthrinse, with and without fluoride, on plaque metabolic acid production and pH after a sucrose challenge. Caries Res. 2004; 38: 537-41.
16. Tomás I, Cousido MC, Tomás M, Limeres J, García-Caballero L, Diz P. In vivo bactericidal effect of 0.2% chlorhexidine but not 0.12% on salivary obligate anaerobes. Arch Oral Biol. 2008; 53: 1186-91.
17. Albertsson WK, Persson A, Lingström P, van Dijken JW. Effects of mouthrinses containing essential oils and alcohol-free chlorhexidine on human plaque acidogenicity. Clin Oral Investig. 2010; 14: 107-12.
18. Dashper SG, Reynolds EC. Characterization of transmembrane movement of glucose and glucose analogs in Streptococcus mutants Ingbritt. J Bacteriol. 1990; 172: 556–63.
19. Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995; 49: 711-45.
20. Marsh PD, Martin MV. Dental plaque. In: Oral microbiology. 3.ed. London: Chapman and Hall; 1992. p.98-132.
21. Landa AS, van der Mei HC, Busscher HJ. Detachment of linking film bacteria from enamel surfaces by oral rinses and penetration of sodium lauryl sulphate through an artificial oral biofilm. Adv Dent Res. 1997; 11: 528-38.
22. Kocak MM, Ozcan S, Kocak S, Topuz O, Erten H. Comparison of the efficacy of three different mouthrinse solutions in decreasing the level of Streptococcus mutans in saliva. Eur J Dent. 2009; 3: 57-61.
23. Arweiler NB, Boehnke N, Sculean A, Hellwig E, Auschill TM. Differences in efficacy of two commercial 0.2% chlorhexidine mouthrinse solutions: a 4-day plaque re-growth study. J Clin Periodontol. 2006; 33: 334-9.
24. Witt JJ, Walters P, Bsoul S, Gibb R, Dunavent J, Putt M. Comparative clinical trial of two antigingivitis mouthrinses. Am J Dent. 2005; 18:15A-17A.
25. Moran J, Addy M, Newcombe R. A 4-day plaque regrowth study comparing an essential oil mouthrinse with a triclosan mouthrinse. J Clin Periodontol. 1997; 24: 636–9.
26. Fine DH, Furgang D, Barnett ML, Drew C, Steinberg L, Charles CH, et al. Effect of an essential oil-containing antiseptic mouthrinse on plaque and salivary Streptococcus mutans levels. J Clin Periodontol. 2000; 7: 157–61.
27. Pan P, Barnett ML, Coelho J, Brogdon C, Finnegan MB. Determination of the in situ bactericidal activity of an essential oil mouthrinse using a vital stain method. J Clin Periodontol. 2000; 27: 256–61.
 Correspondence:
Correspondence:
Geraldo Thedei Jr.
Pró-reitoria de Pesquisa, Pós-Graduação e Extensão - Universidade de Uberaba.
Avenida Nenê Sabino, 1801 Bloco R.
Bairro Universitário Uberaba - MG - CEP 38055-500
E-mail: geraldo.thedei@uniube.br
Received for publication: November 16, 2010
Accepted: March 22, 2011













