Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.10 no.2 Piracicaba Abr./Jun. 2011
ORIGINAL ARTICLE
Comparison of halitosis parameters and sialometry between patients subjected to head and neck radiotherapy and patients with periodontal disease
Danielle Frota de AlbuquerqueI; Elen de Souza TolentinoII; Flávio Monteiro AmadoIII; Cazuo ArakawaIV; Luiz Eduardo Montenegro ChinellatoV
I MSc in Stomatology, Department of Stomatology, Bauru Dental School, University of São Paulo, Brazil
II PhD student, Bauru Dental School, Department of Stomatology, University of São Paulo, Brazil
III PhD in Stomatology, Bauru Dental School, Department of Stomatology, University of São Paulo, Brazill
IV MD, Radiotherapy specialist, Department of Radiotherapy, Manoel de Abreu Hospital, Bauru, SP, Brazil
V PhD, Professor, Bauru Dental School, Department of Stomatology, University of São Paulo, Brazil
ABSTRACT
Aim: The aim of this study was to evaluate halitosis parameters and sialometry in patients subjected to head and neck radiotherapy compared to patients with periodontal disease, establishing a relationship between oral concentration of volatile sulfur compounds (VSCs) and tongue coating presence, salivary flow rate and BANATM test. Methods: Thirty-eight patients were examined and divided into 2 groups: group I: patients with chronic generalized periodontal disease previously diagnosed and not treated; group II: patients subjected to head and neck radiotherapy. All volunteers were subjected to halitosis measurements through a sulphide monitor, evaluation of tongue coating weight, stimulated and non-stimulated sialometry and BANATM test. Results: The results were analyzed by analysis of the variance, Pearson's correlation coefficient and Student's t-test, showing that both groups presented halitosis. There was also a relationship between tongue coating presence and VSC levels in both groups and the irradiated patients showed lower salivary flow rates. Conclusions: Under the tested conditions, it may be concluded that halitosis can be considered as an adverse effect of radiotherapy, associated with low salivary flow and poor oral health, which seems to be the main contribution to bad breath, since patients with periodontal disease also showed halitosis.
Keywords: halitosis, radiotherapy, head and neck neoplasms, periodontitis.
Introduction
Radiotherapy, alone or associated with surgery or chemotherapy, is the therapeutic method indicated in cases of oral cancer and has produced a significant increase in cure rates for several malignancies of the head and neck1. The adverse effects of head and neck radiotherapy are very important for the dental surgeon, who has a key role in preventing and/or minimizing their occurrence. Salivarydegeneration caused by radiotherapy commonly result in reduction of the saliva production2-5.
Halitosis is highly associated with the amount of saliva6. It has been shown a relationship between hyposalivation and radiotherapy, as well as between halitosis and periodontal disease. However, the association among these variables is not well known. There is only one study which established a correlation between halitosis and head and neck radiotherapy, through evaluation of halitosis and sialometry in patients who had undergone radiotherapy when compared to healthy and non-irradiated individuals7. Howsoever, there are no studies comparing halitosis between irradiated patients and patients with periodontal disease.
The aim of this study was to establish a correlation between halitosis and head and neck radiotherapy, through evaluation of halitosis and sialometry in patients who had undergone radiotherapy in comparison to patients with periodontal disease.
Material and methods
This study was approved by the Human Research Ethics Committee of Bauru Dental School, University of São Paulo, Brazil (process #104/2005) and is in accordance with the Helsinki Declaration of 1975, revised in 1983. All volunteers signed an informed consent form. The sample was composed of 38 patients, divided into two groups: Group I: 13 patients from the screening sector of the Bauru Dental School , with generalized chronic periodontal disease previously diagnosed but not treated; Group II: 25 volunteers selected among patients from Manoel de Abreu Hospital, referenced for cancer treatment in the city of Bauru, SP, Brazil. The sample size was justified by the fact of many patients have not finished the radiotherapy treatment at the moment of the consultation and only volunteers with the treatment completed were included in the study. In Group I, patients with localized or treated periodontal disease were excluded, which reduced the initial sample size. All patients were selected among a population of each Institution, both with spontaneous demand. Careful exam and screening of these patients according to the exclusion and inclusion criteria led to the reduction of the sample; however, according to the statistician responsible for this study, the sample size was appropriated.
Patients of Group I presented periodontal pockets > 5 mm in all molars and incisors, bleeding on probing and generalized vertical bone loss observed radiographically. The diagnosis of these patients was chronic generalized periodontal disease. Patients of Group II were subjected to radiotherapy as the main or complementary treatment of head and neck tumors. The radiation area covered at least one of the major salivary glands or part of them. The radiotherapy treatment of all patients had already been concluded and they were under continuous monitored from 1 to 6 months after the last radiotherapy session. It is important to emphasize that all patients of Group II were periodontally healthy, that is, they did not present bleeding on probing or periodontal pockets > 4 mm). Also, the sequelae of the radiotherapy treatment (e.g.: radiation caries and mucositis) were treated in the period between the radiotherapy and this research. The management of the patients was similar to a previous study7. All patients underwent two appointments conducted by the same examiner. In the first one, the patients received some guidelines for the procedures to be carried out in a further moment: avoid spicy and/ or flavored food 24 h before the clinical appointment; avoid brushing the teeth, using dental floss, chewing gum, drinking alcoholic drinks and smoking 3 h before the clinical appointment and not being using perfume at the moment of the appointment. Halimetry Evaluation of tongue coating and new halimetry measurements BANATM Test (benzoyl-DL-arginine-2 napthylamide) Sialometry Radiotherapy treatment protocol Statistical analysis
Results
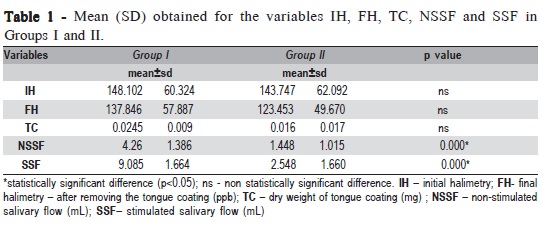
Group I presented lower initial halimetry average (148.102 ppb) when compared to Group II (143.747 ppb), with no statistically significant difference (Table 1). The dry weight of tongue coating in Group II (0.016mg) was smaller than in Group I (0.0245 mg), but this difference was not significant (Table 1). Group II showed a decrease in salivary flow, in stimulated and non-stimulated sialometry, with a statistically significant difference (p<0.001) (Table 1).
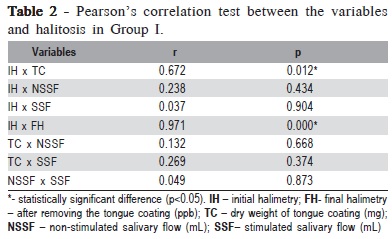
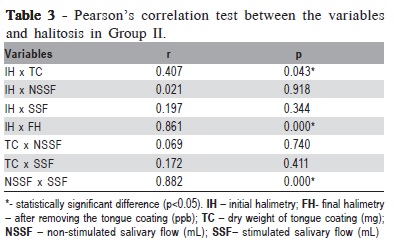
In the evaluation of the correlation between the studied variables and halitosis, in Group I this correlation was noted between initial halimetry and presence of tongue coating (p=0.012) and between initial and final halimetry (p<0.001) (Table 2.). Group II, showed a significant correlation between initial halimetry and presence of tongue coating (p=0.043), between initial and final halimetry (p<0.001) and between stimulated and non-stimulated salivary flow (p<0.001) (Table 3).
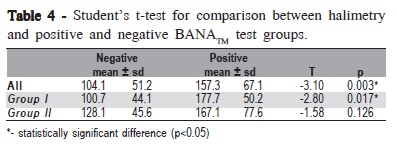
In both groups, the number of negative BANATM tests (n=25) was greater than the positive results (n=23), with a statistically significant difference among the negative and positive results and halimetry in Group I (p=0.017) and when the entire sample was analyzed together (p=0.003) (Table 4).
Discussion
Regarding oral halimetry, Group I presented lower initial average when compared to Group II (Table 1), but without statistically significant difference. However, in the present study, the use of the sulfide monitor enabled the evaluation only of the relation between VSCs and halitosis. This is a limitation of methodology since bad breath is caused by other volatile organic compounds and other gases8.
Final halimetry was performed soon after tongue cleaning. A previous study7 demonstrated that the values of halimetry can decrease substantially immediately after the tongue cleaning. It is known that tongue coating is an important etiological factor of halitosis9. Waler10 showed that the largest production of VSCs is in the tongue dorsum. Yaegaki and Sanada11 reported that removing tongue coating reduced by 50% the production of VSCs. Albuquerque et al.7 found a relationship between presence of tongue coating and VSCs levels, with a decrease in the values of halimetry after removing tongue coating. Boever and Loesche12 noticed that the score of oral breath was highly connected to the odor of the tongue, presence and extension of coating. In this work, the values of initial halimetry were greater than final halimetry in both groups. However, there was no statistically significant difference (Table 1). Since the tongue cleaner shows a higher percentage of reduction of VSCs than the tooth brush13, it justifies the use of this tool in the methodology of this research. In our study, the amount of tongue coating was evaluated quantitatively after drying, confirming methodologies used by other studies7,11,14. The dry weight of tongue coating in patients with periodontal disease was greater than in the irradiated patients, but the difference was not statistically significant. This finding is a little surprising because it is well established that the tongue coating is a result of the hypossalivation7. Salivary reduction provides the implantation of the tongue coating because the saliva becomes more viscous, with higher amounts of mucin, increasing the adherence of microorganisms and epithelial debris in the tongue. As the bacteria present in the tongue coating and in the periodontal pockets are similar (anaerobic proteolytic), colonization of the tongue can be favored in patients with periodontal disease. However, it does not explain the higher amounts of tongue coating in Group I than in Group II, since irradiated patients have an outstanding decrease in salivary flow, the main etiological factor of tongue coating. Perhaps the poor hygiene, which is common in patients with periodontal disease, can explain this finding of the present work.
Regarding salivary flow, there was significant difference between the groups in stimulated and non-stimulated sialometry (Table 1). All patients in Group II showed a decrease in the amount of saliva. The value of 0.1 mL/min for salivary flow without stimulus was considered severe glandular hypofunction. There was a decrease in sialometry without stimulus from group II of 66.03% compared to Group I. Albuquerque et al.7 found a decrease in sialometry without stimulus in irradiated patients of 56.51% when compared to healthy patients.
In Group I, correlation between the studied variables and halitosis was noted between initial halimetry and presence of tongue coating and between stimulated and non-stimulated salivary flow (Table 2). In group II, a significant correlation was observed between initial and final halimetry, between initial halimetry and presence of tongue coating and between stimulated and non-stimulated salivary flow (Table 3).
The BANATM test - an enzymatic method used as an indicator of the presence of microorganisms responsible for periodontal diseases - was performed in Groups I and II. Similarly to a previous study7, in both groups the number of negative tests was greater than positive results. In Table 4, it is noted that there was a significant difference among the negative and positive results and halimetry in Group I and when the entire sample was together. Some authors have not noted relation between the levels of VSCs and the results of lingual BANATM test15. According to Monteiro-Amado et al.14, there is not a relation between the value of BANATM test and the values of halimetry.
Irradiated patients did not have main complaint of halitosis. However, when they were questioned about oral bad breath, the majority answered that felt it. Patients with periodontal disease did not ask for treatment of halitosis, but they had complaints of bad breath and presented indication for periodontal treatment.
According to Conceição et al.16, halimetry above 100 ppb can be considered as halitosis. In this study, all evaluated patients, of both groups, presented halitosis, since the values of initial and final halimetry were greater than 100 ppb. However, there was no statistically significant difference between the groups, confirming the fact that the oral health condition is an important etiological factor of halitosis, and bad breath is strongly associated with periodontal disease. It is known that saliva incubation of patients with periodontal disease produces VSCs quickly, inducing an intense bad breath. The saliva of these patients produces more VSCs than the saliva of healthy people. Halitosis may occur in any individual, but it is emphasized when inflammatory and degenerative processes are present. For example, gingivitis and periodontitis are almost always associated with halitosis, which corroborates the findings of the present study.
The present work is a continuation of a previous study, which detected halitosis and hyposalivation in irradiated patients when comparing them with healthy people7. It is believed that the comparison of periodontally healthy irradiated patients, which recognizably have halitosis,) and patients with periodontal disease can show the true degrees of halitosis in each group as well as demonstrate that both conditions play important roles in bad breath development.
It is known that hyposalivation is a definitive sequel of head and neck radiotherapy and that this condition plays a keyrule in halitosis pathogenesis6. We believe that the significant reduction in the amount of saliva is the most important parameter in the bad breath development in irradiated patients, since, in this research, they had a satisfactory oral condition. On the other hand, the significant differences among the negative and positive results and halimetry of the BANATM tests in Group I show the substantial role of periodontopathogenic microorganisms in the development of halitosis in patients periodontally affected. Additionally, despite the lack of statistical significance, the dry weight of tongue coating in Group I was greater than in Group II, showing a close relationship between periodontitis and tongue coating, since the bacteria that colonize the tongue and periodontal pockets are the same. For these reasons, we agree that the most expressive factors that influence the halitosis formation in patients with periodontal disease are tongue coating and presence of periodontopathogenic microorganisms.
Summarizing our findings, halitosis was detected in patients with periodontal disease and patients subjected to head and neck radiotherapy and that there was relation between presence of tongue coating and VSCs levels in the studied groups. There was a significant decrease in the values of halimetry after removing tongue coating in both groups. Also, stimulated and non-stimulated salivary flow was extremely reduced in irradiated patients. According to the results obtained under the tested conditions, it is possible to conclude that halitosis is an adverse effect of head and neck radiotherapy and is strongly associated with periodontal disease.
Acknowledgements
The authors would like to thank Professor José Roberto Lauris for the support in the statistical analysis of this study. This investigation was supported by CAPES.
References
1. Otmani N. Oral and maxillofacial side effects of radiation therapy on children. Clin Pract. 2007; 73: 257-61. [ Links ]
2. Prott FJ, Handschel J, Micke O, Sunderkotter C, Meyer U, Piffko J. Long-term alterations of oral mucosa in radiotherapy patients. Int J Rad Oncol Biol Phys. 2002; 54: 203-10.
3. Ko C, Citrin D. Radiotherapy for the management of locally advanced squamous cell carcinoma of the head and neck. Oral Dis. 2009; 15: 121-32.
4. Bomeli SR, Desai SC, Johnson JT, Walvekar RR. Management of salivary flow in head and neck cancer patients – a systematic review. Oral Oncol. 2008; 44: 1000-8.
5. Sciubba JJ, Goldenberg D. Oral complications of radiotherapy. Lancet Oncol. 2006; 7: 175-83.
6. Möller P, Perrier M, Ozsahin M, Monnier P. A prospective study of salivary gland function in patients undergoing radiotherapy for squamous cell carcinoma of the oropharynx. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 97: 173-89.
7. Albuquerque DF, Tolentino ES, Monteiro-Amado F, Arakawa C, Chinellato LEM. Evaluation of halitosis and sialometry in patients subjected to head and neck radiotherapy. Med Oral Patol Oral Cir Bucal. 2010; 15: e850-4.
8. Goldberg S, Cardash H, Browning H, 3rd, Sahly H, Rosenberg M. Isolation of Enterobacteriaceae from the mouth and potential association with malodor. J Dent Res. 1997; 76: 1770-5.
9. Rosenberg M. Clinical assessment of bad breath: current concepts. J Am Dent Assoc. 1996; 127: 475-82.
10. Waler SM. On the transformation of sulfur-containing amino acids and peptides to volatile sulfur compounds (VSC) in the human mouth. Eur J Oral Sci. 1997; 105: 534-7.
11. Yaegaki K, Sanada K. Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with periodontal disease. J Periodontal Res. 1992; 27: 233-8.
12. Boever EH, Loesche WJ. Assessing the contribution of anaerobic microflora of the tongue to oral malodor. J Am Dent Assoc. 1995; 126: 1384-93.
13. Seemann R, Kison A, Bizhang M, Zimmer S. Effectiveness of mechanical tongue cleaning on oral levels of volatile sulfur compounds. J Am Dent Assoc. 2001; 132: 1263-8.
14. Monteiro-Amado F, Chinellato LE, de Rezende ML. Evaluation of oral and nasal halitosis parameters in patients with repaired cleft lip and/or palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005; 100: 682- 7.
15. Willis CL, Gibson GR, Holt J, Allison C. Negative correlation between oral malodour and numbers and activities of sulphate-reducing bacteria in the human mouth. Arch Oral Biol. 1999; 44: 665-70.
16. Conceição MD, Marocchio L, Tarzia O. Evaluation of a new mouthwash on caseous formation. Braz J Otorhinolaryngol. 2008; 74: 61-7.
 Correspondence:
Correspondence:
Elen de Souza Tolentino
Faculdade de Odontologia de Bauru-USP
Departamento de Estomatologia
Alameda Dr. Octávio Pinheiro Brisola, 9 - 75
Vila Universitária - CEP 17012-901 Bauru - SP - Brasil
Phone: (14) 3235-8000 / Fax: (14) 3223-4679
E-mail: elen_tolentino@hotmail.com
Received for publication: January 28, 2011
Accepted: May 20, 2011













