Brazilian Journal of Oral Sciences
ISSN 1677-3225
Braz. J. Oral Sci. vol.10 no.2 Piracicaba abr./jun. 2011
ORIGINAL ARTICLE
Temporomandibular disorder and generalized joint hypermobility: electromyographic analysis of the masticatory muscles
Fernanda PasinatoI; Juliana Alves SouzaII; Eliane Castilhos Rodrigues CorrêaIII; Ana Maria Toniolo da SilvaIV
I MS, Assistant Professor of Physical Therapy, Federal University of Pampa, Brazil
IIMS, Physical therapist, University Hospital of Santa Maria, Brazil
IIIPhD, Adjunt Professor, Department of Physical therapist, Federal University of Santa Maria; Graduate Program in Human Communication Disorders, Federal University of Santa Maria, Brazil
IVPhD, Associate Professor, Department of Speech Pathology, Federal University of Santa Maria; Graduate Program in Human Communication Disorders, Federal University of Santa Maria, Brazil
ABSTRACT
Aim: This study aimed to verify the presence of generalized joint hypermobility (GHJ) in individuals with temporomandibular disorders (TMD) and asymptomatic individuals and to compare the activity of their masticatory muscles. Methods: 61 female patients aged 18 to 35 years were evaluated: 34 with diagnosis of TMD by the Research Diagnostic Criteria for Temporomandibular Disorders constituted the TMD group and 27 constituted the asymptomatic group. The subgroups were classified according to the presence of GJH by the Beighton score. Electromyographic recordings of the masseter and anterior temporal muscles were acquired bilaterally at mandibular rest and in maximal intercuspal position. Results: GJH was present in 64.71% of the individuals with TMD and in 40.74% of the asymptomatic individuals. The electrical activity was significantly higher in the right masseter (p = 0.0111), left masseter (p = 0.0007) and right temporal (p = 0.0046) in the patients with TMD than in the asymptomatic individuals. The activity of the left masseter muscle was significantly higher (p=0.0072) in the volunteers with TMD and GJH compared with in the individuals with TMD but without hypermobility. Also, the right temporal muscle showed higher activity in subjects with GJH and TMD compared with asymptomatic individuals without hypermobility (p=0.0248). Conclusions: The electrical activity was higher at mandibular rest in TMD and TMD/ GJH patients. This result suggests that these muscles need to be recruited for the joint stabilization due to the low ligamentar resistance and a possible proprioceptive deficit. This recruitment appears to occur asymmetric and variedly among all muscles involved in this stabilization, which could compensate for the low ligamentar competence and a possible proprioceptive deficit in individuals with GJH. Both TMD and GJH seem to have influenced the muscular activity.
Keywords: temporomandibular disorder, hypermobility, joint instability, electromyography, masticatory muscles.
Introduction
The association between generalized joint hypermobility (GJH) and temporomandibular disorders (TMD) has been addressed by several studies1-8. It is believed that the temporomandibular joint is one of the hypermobile joints. On the other hand, the results of the studies regarding this relationship are conflicting generally due to discrepancies in the sampling and methodology used.
GJH is characterized by the excessive range of motion of several joints due to ligamentous laxity, and may be associated with chronic and recurrent musculoskeletal symptoms in patients without any visible rheumatologic pathology9. The alteration of proprioceptive acuity may be the cause or the effect of hypermobility, encouraging the adoption of biomechanically inadequate postures and consequently joint trauma. Moreover, loose ligaments produce down-regulation related to muscle stretch receptors, reducing the proprioception10. Associated to these changes, joint instability in GJH patients can alter the modulation of muscle contraction.
Bird11 considers that GJH depends not only on ligament laxity, but also on skin, blood vessels and adjacent muscle tissue that allow the occurrence of this phenomenon. Simmonds and Keer12 reported that usually there is little muscle definition and the rest tone is low even when the individual is submitted to proper training.
It is possible to consider that the proprioceptive changes in the modulation of muscle contraction in individuals with GJH may influence the pattern of electrical activity on masticatory muscles associated to a clinical state of TMD.
In recent years, several studies involving electromyography of masticatory muscles have been performed13-16. These studies have demonstrated that there is higher electrical activity of the masticatory muscles at rest, especially the anterior temporal, in individuals with TMD. Such condition can be explained by the higher activity required to keep the mandibular rest position in patients with TMD and myofascial pain14.
Although there are studies about GJH and TMD, there is neither consensus about their association nor studies that investigate the pattern of electrical activity of masticatory muscles in individuals with both conditions. Understanding the musculoskeletal and ligament system as a complex formed by muscular and fascial chains linked, it should be investigated whether the presence of GJH influence the electrical activity of masticatory muscles.
Searching individuals with joint hypermobility is essential to identify patients with potential risk to develop certain injuries and prevent them. The pathogenetic mechanisms for the development of joint symptoms in the joint hypermobility are not well defined and may be related to excessive and/or inadequate use of the joint, which may not be a causative factor of TMD but would predispose to its occurrence17. Therefore, this study aimed to verify the presence of GJH in individuals with TMD and asymptomatic individuals and to compare the electrical activity of their masticatory muscles.
Material and methods
Subjects
Sixty-one female volunteers aged between 18 and 35 participated in the study and were distributed in two groups.The Study Group (SG) was composed by 34 individuals presenting signs and chronic symptoms of TMD (for a period superior to 6 months) who sought the discipline of prosthodontics occlusion of the Federal University of Santa Maria or the researchers in response to the research advertising in print and electronic media. The SG volunteers had one or more diagnoses according to the Research Diagnostic Criteria for TMD (RDC/TMD). The Control Group (CG) was composed by 27 asymptomatic volunteers who did not present any signs and symptoms of TMD or bruxism, based on history and clinical signs according to the RDC/TMD.
Individuals were excluded from the study with neuropsychomotor impairment, history of orthopedic trauma or malformation of face; systemic or rheumatologic disease in physical, dental or speech therapy prior to the study, or using any medication.
The study was approved by the University Ethics Committee under number 0281.0.243.000-08. All participants were informed about the nature and objectives of the study and signed an informed consent form before participating in the research.
Evaluation Procedures
The RDC/TMD classify TMD diagnoses into three groups: I) Muscular (only myofascial pain or myofascial pain with limited opening); II) Disc Displacement (with or without reduction with limited opening or without reduction without limited opening); III) Arthralgia and TMJ osteoarthritis/ osteoarthrosis18.
GJH has been evaluated by the criteria of Carter and Wilkinson modified by Beighton19, which have been used in several studies1-2,4-9 on hypermobility. The Beighton score examines 9 joints on 5 tests: apposition of thumb to the anterior forearm until they touch; passive dorsiflexion of the little finger until it is parallel to the forearm; trunk flexion with knees fully extended so palms touch the floor; and elbow and knee hyperextension beyond 10 degrees. Each joint with hypermobility scores one point. GJH is diagnosed with a score equal or greater than 4. GJH is considered moderate (4-6 points) and severe (7-9 points)4.
SG and CG groups were subdivided after this examination: SG with GJH (SGH), SG without GJH (SGN), CG with GJH (CGH) and CG without GJH (CGN).
The electromyographic (EMG) exam of masticatory muscles (masseter and anterior temporal) was carried out with an electromyography of eight channels with analog-digital conversion board of 16-bit model CAD 10/26, sampling frequency of 2 KHz, Butterworth filter with high-pass cut-off frequency of 10Hz and low-pass of 1000Hz (Lynx Electronic Technology Ltda). The acquisition software BioInspector developed by Lynx Electronic Technology Ltda was used.
Before collecting the EMG signal, the skin impedance was reduced by cleaning with isopropyl alcohol swab 70° (ISEK - International Society of Electromyography and Kinesiology). Double-junction Ag/AgCl electrodes (Hall Indústria e Comércio Ltda) with circular shape, fixed distance of 20 mm, diameter of 10 mm, 2 mm contact surface, 20x gain, input impedance of 10 GÙ and common mode rejection ratio >100 dB were connected to active preamps with differential input (PA1020) from Lynx Electronic Technology Ltda. Surface electrodes were fixed in the region of the muscle belly13 and a reference electrode was fixed on the region of the sternum bone20. For collecting and storing data a notebook DELL Latitude D520, Intel (R) Celeron (R) M CPU 430@1.73GHz, real speed of 1.69 GHz, 1536 MB of RAM memory, Microsoft Windows XP Professional Operating System Version 5.1.2600, was used disconnected from the electrical grid.
The muscular electric activity was recorded bilaterally in two mandibular positions21:
1) Mandibular physiologic rest position: EMG signal collected for 10 s.
2) Maximal intercuspal: oriented under the examiner's verbal command "clench, clench, clench". The signal was collected for 5 s, three times, with the material Parafilm "M"® (Chicago, IL, USA) located between the premolars, the maxillary 1st and 2nd molars. A 2-min between each signal collecting was maintained in order to minimize the effects of muscle fatigue.
The recordings was accomplished in the orthostatic posture, with the subject barefoot, feet parallel, arms along the body and stare at a target at eye level. The signal acquisition was carried out three times in each situation and the one with the best quality and lowest presence of noise was selected. EMG amplitude values were quantified using the Root Mean Square (RMS) and expressed in microvolts (μV)14,21-23. This measure is recommended to represent EMG amplitude of static contractions, such as isometric – maximal intercuspal24. The software AqDAnalisys 7.0 (Lynx Electronic Technology Ltda) was used for the signal processing.
Statistical analysis
Statistical analysis was performed by the software Statsoft STATISTICA 7.1. The data showed normal distribution by Shapiro Wilk test (p <0.05). The difference between EMG activity of masticatory muscles in SG and CG was analyzed by the Student's t-test for independent samples. Comparison of EMG activity among the subgroups with and without GJH was verified by one-way ANOVA and Tukey's test was used for multiple comparisons when significant difference was detected. To verify the difference in the frequency of GJH between groups the chi-square and Fisher's tests were used. The level of significance was set at p< 0.05 for all analyses.
Results
From the evaluation of TMD and hypermobility, participants were distributed in a SG (n=34) and a CG (n=27), as described before. These two groups were subdivided into four subgroups: SG with GJH (SGH), n = 22; SG without GJH (SGN), n = 12; CG with GJH (CGH), n = 11; and CG without GJH (CGN), n = 16.
The mean age of the participants in the SG was 25.7 ± 5.0 years old and in the control group was 22.4 ± 2.3 years old.
The SG was characterized by individuals who had signs and/or chronic symptoms of TMD (more than 6 months). Among these symptoms, all participants reported complaints of bruxism concentric and/or eccentric.
Among subjects with TMD, 64.71% of participants had hypermobility and 35.29% had normal mobility. In the control group, 40.74% had hypermobility and 59.25% showed normal mobility. The chi-square test did not find statistically significant different between the groups (p = 0.0621).
Besides, the frequency of severe hypermobility (7-9 points on the Beighton score) was higher in the SG (23.53%) than in the CG (7.41%). The percentage of severity degrees of GJH in both groups were evaluated using the chi-square and Fisher's tests. There were no statistically significant differences between groups for moderate (p = 0.5301) and for severe hypermobility (p = 0.0878).
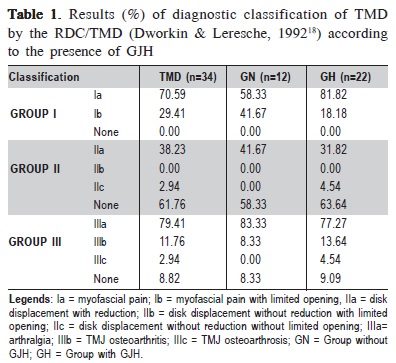
All TMD individuals were diagnosed with myofascial pain (Group I), 41% had a diagnosis of disc disorders (Group II) and 91% had some type of joint involvement (Group III), especially arthralgia (79.41%).
When the participants were subdivided according to GJH, there was a higher percentage of myofascial pain without limited mouth opening (Ia) in individuals with GJH (81.82%) compared with those without GJH (58.33%). This difference was not significant in the chi-square test (p = 0.2468). Disc displacement with reduction (IIa) was diagnosed in 31.82% of hypermobility and 41.67% of normal joint mobility participants. The diagnosis of arthralgia (IIIa) showed high percentages in both groups (81.82% and 83.33% in the groups with and without GJH, respectively) (Table 1.)
The results of EMG signals of masticatory muscles (masseter and anterior temporal) of the SG and CG are shown in Table 2.
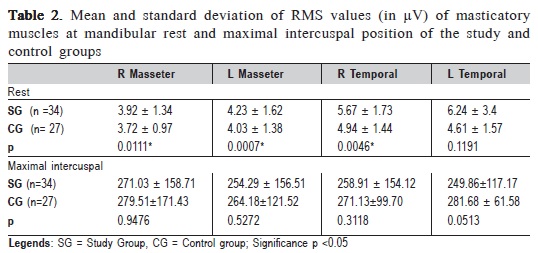
When comparing SG and CG, it was observed a higher electrical activity at rest physiologic mandibular in the temporal compared with the masseter muscles, in individuals with TMD. A statistically significant difference (Students t test) was found for right temporal and masseter muscles. There was no difference in EMG activity between groups during the maximal intercuspal position.
Mean and standard deviations of RMS values (in µV) of masticatory muscles at rest physiologic mandibular of the volunteers, classified according to the presence of GJH, are shown in Table 3.
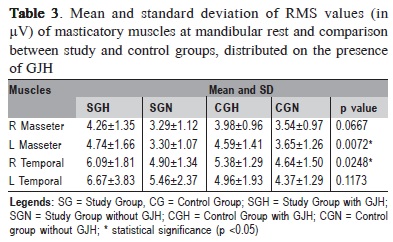
In the comparison between groups by one-way ANOVA, statistically significant differences were observed for the left masseter and right temporal muscles. The Tukey's test revealed the left masseter muscle was significantly more active in subjects with TMD and GJH (SGH) compared with patients with TMD and normal joint mobility (SGN). Also the right temporalis muscle showed a value of RMS significantly higher in SGH compared with CGN. Yet, there was increased electrical activity in the temporalis muscles, with levels of hyperactivity, mainly in SG and SGH.
There were no statistically significant differences in the EMG analysis of masticatory muscles during maximal intercuspal among the individuals with normal mobility and with GJH.
Discussion
The association between TMD and GJH has been investigated in several studies1-8 with inconclusive results.
In the present study, GJH reached high percentages in both groups, even higher in the individuals with TMD (64.71%). However, there was no statistically significant difference in the incidence and severity of GJH between the study and control groups. This result agrees with Conti et al.9 who did not observed differences in the incidence of GJH in symptomatic and asymptomatic groups for TMD, despite the high percentages in both groups. Some authors1,7,25 found an association between TMD and GJH, with higher scores of hypermobility in symptomatic individuals.
Multiple diagnoses according to RDC/TMD were present in most individuals with TMD. However, disc disorders diagnoses were not more frequent in the GJH patients evaluated in this study than in the normal joint mobility, agreeing with Conti et al9 and Saez-Yuguero et al.25. Intraarticular dysfunctions have been correlated with GJH and TMJ hypermobility1,6, since an excessive movement of the mandibular condyle beyond the articular eminence and the ligaments laxity would facilitate intra-articular joint disc displacements.
However, in studies that considered older individuals26-27, the diagnosis of disc disorders was more frequent and there are reports1 that the diagnosis of disc displacement increases with age. In the present study, it can be considered that the low mean age of the evaluated volunteers may have contributed for this result. Furthermore, TMD individuals showed high incidence of arthralgia, regardless of the joint mobility condition, which may indicate an earlier stage of joint damage considering the young group studied.
High percentage of myofascial pain without limited mouth opening was observed in individuals with GJH (81.82%) compared with those without GJH (58.33%), but there was no significant difference between them. Hirsh et al.8 confirmed the lower risk of GJH subjects to develop limited mouth opening. The preservation of the mandibular motion range within physiological parameters, in these individuals, may lead to a low functional repercussion and late diagnosis.
On the EMG evaluation of masticatory muscles of TMD individuals and control group, there was significant higher electrical activity at rest physiologic mandibular in all assessed muscles, except the left temporal in the TMD group. Several authors14-16,28 confirmed the higher electrical activity of masticatory muscles at rest, especially the anterior temporal, in patients with TMD. This behavior is explained by the need for greater muscle recruitment in patients with TMD and myofascial pain at mandibular rest14. In the present study, it was also observed a predominance of electrical activity of the temporal over the masseter muscles, with levels of hyperactivity in SG compared with the CG.
Rodrigues-Bigaton et al.15 also observed a higher electrical activity of masticatory muscles at rest in TMD patients compared with controls. However, this increase did not reach levels of muscular hyperactivity, but it was considered by the authors as a suggestive sign of TMD. The present study observed levels below of the hyperactivity - 5 μV - for all muscles studied only in CGN. It suggests that both TMD and hypermobility contribute to the muscle hyperactivity.
Multiple comparisons in the rest physiologic mandibular activity found significantly higher levels in the left masseter muscle when comparing individuals with TMD and GJH (SGH) and TMD without GJH (SGN). Besides, the right temporal muscle was more active in individuals with TMD and GJH (SGH) compared with controls without GJH (CGN).
The literature has been reporting higher activity at rest in the masticatory muscles in individuals with TMD14-15,28. The results of this present study indicate that the GJH may contribute to higher EMG activity in these muscles.
Considering that in individuals with TMD associated with GJH the mandibular rest position, which should be maintained by the viscoelasticity of muscles, ligaments, articular capsule and the subatmospheric pressure of mouth13, is hampered by the reduction of ligament resistance10, it is assumed that the masticatory muscles may be recruited to participate in the TMJ stabilization. This recruitment appears to occur asymmetric and variedly among all muscles involved in this stabilization, which could compensate the low ligamentar competence and a possible proprioceptive deficit in individuals with GJH. According to Ferrell et al.29, besides the excessive motion range of some joints, the only recognized neurophysiologic abnormalities in individuals suffering from GJH were the proprioceptive deficit.
On the EMG evaluation of masseter and temporal muscles during maximal intercuspal it was not observed significant differences between groups. These results agree with those of Rodrigues et al.30, who did not find differences between TMD and control groups during maximal intercuspal. On the other hand, other study14 observed lower EMG activity in patients with TMD.
In this study, the masticatory muscles of individuals with TMD and GJH presented a different and non-specific activation pattern. Thus, it is assumed that TMD demands greater muscle recruitment and GJH determines difficulty in modulating the muscle contraction due to joint instability associated to a proprioceptive deficit.
No studies were found in the literature associating EMG variables to GJH. However, since GJH is a feature often found in individuals with TMD, it is important to study which effects this phenomenon can cause in the masticatory muscles. As this topic has not yet been explored, further studies are needed to generalize the obtained results.
References
1. Westling L, Mattiasson A. General joint hypermobility and temporomandibular joint derangement in adolescents. Ann Rheum Dis. 1992; 51: 87-90. [ Links ]
2. Perrini F, Tallents RH, Katzberg RW, Ribeiro RF, Kyrkanides S, Moss ME. Generalized joint laxity and temporomandibular disorders. J Orofac Pain. 1997; 11: 215-21.
3. Winocur E, Gavish A, Halachmi M, Bloom A, Gazit E. Generalized joint laxity and its relation with oral habits and temporomandibular disorders in adolescent girls. J Oral Rehabil. 2000; 27: 614-22.
4. Silveira EB, Rocabado M, Russo AK, Cogo JC, Osorio RA. Incidence of systemic joint hypermobility and temporomandibular joint hypermobility in pregnancy. Cranio. 2005; 23: 138-43.
5. De Coster PJ, Van Den Berghe LI, Martens LC. Generalized joint hypermobility and temporomandibular disorders: inherited connective tissue disease as a model with maximum expression. J Orofac Pain. 2005; 19: 47-57.
6. Kavuncu V, Sahin S, Kamanli A, Karan A, Aksoy C. The role of systemic hypermobility and condylar hypermobility in temporomandibular joint dysfunction syndrome. Rheumatol Int. 2006; 26: 257-60.
7. Deodato F, Trusendi R, Giorgetti R, Scalese MU. Predisposition for temporomandibular joint disorders: loose ligaments. Cranio. 2006; 24: 179-83.
8. Hirsch C, John MT, Stang A. Association between generalized joint hypermobility and signs and diagnoses of temporomandibular disorders. Eur J Oral Sci. 2008; 116: 525-30.
9. Conti PCR, Miranda JES, Araujo CRP. Relationship between systematic joint laxity, TMJ hypertranslation and intra-articular disorders. Cranio. 2000; 18: 192-7.
10. Egri D, Yoshinari NH. Generalized joint hypermobility. Rev Bras Reumatol. 1999; 39: 231-6.
11. Bird HA. British Society for Rheumatology Meeting report – Special interest group for joint hypermobility. Br J Rheumatol. 1993; 32: 81.
12. Simmonds JV, Keer RJ. Hypermobility and the hypermobility syndrome. Man Ther. 2007; 12: 298-309.
13. Faria CRS; Bérzin F. Electromyographic study of the temporal, masseter and suprahyoid muscles in the mandibular rest position. J Oral Rehabil. 1998; 25: 776-80.
14. Pinho JC, Caldas FM, Mora MJ, Santana-Penín U. Electromyographic activity in patients with temporomandibular disorders. J Oral Rehabil. 2000; 27: 985-90.
15. Rodrigues-Bigaton D, Berto R, Oliveira AS, Berzin F. Does masticatory muscle hyperactivity occur in individuals presenting temporomandibular disorders? Braz J Oral Sci. 2008; 7: 1497-501.
16. Castroflorio T, Bracco P, Farina D. Surface electromyography in the assessment of jaw elevator muscles. J Oral Rehabil. 2008; 35; 638-45.
17. Salomão EC, Barbosa JS. Association between generalized joint hypermobility and craniomandibular. dysfunction Reabilitar. 2003; 5: 32-7.
18. Dworkin SF, Leresche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J. Craniomandib. Disord. 1992; 6: 301-55.
19. Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann Rheum Dis. 1973; 32: 413-8.
20. Cram JR, Kasman GS, Holtz J. Introduction to Surface Electromyography.Gaithersburg, Maryland: An Aspen Publication; 1998.
21. Pedroni CR. Diagnostic application of surface electromyography for temporomandibular disorders [thesis] Piracicaba: FOP-Unicamp; 2007.
22. Ribeiro EC, Marchiori SC, Da Silva AM. Electromyographic muscle EMG activity in mouth and nasal breathing children. Cranio. 2004; 22: 145-50.
23. Tecco S, Epifania E, Festa F. An electromyographic evaluation of bilateral symmetry of masticatory, neck and trunk muscles activity in patients wearing a positioned. J Oral Rehabil. 2008; 35: 433-9.
24. [SENIAM 7 ] The state of the Art on Signal Processing Methods for Surface ElectroMyoGraphy, deliverable of the SENIAM project. Hermens HJ, Merletti R, Freriks B, editors. Freriks, Roessingh Research and Development; 1999.
25. Saéz-Yuguero Mdel R, Linares-Tovar E, Calvo-Guirado JL, Bermejo- Fenoll A, Rodríguez-Lozano FJ. Joint hypermobility and disk displacement confirmed by magnetic resonance imaging: A study of women with temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107: 54-7.
26. Manfredini GC, Bosco M. Research diagnostic criteria for temporomandibular disorders (RDC/TMD) axis I diagnosis in Italian patient population. J Oral Rehabil. 2006; 33: 551-8.
27. Tartaglia GM, Moreira Rodrigues da Silva MA, Bottini S, Sforza C, Ferrario VF. Masticatory muscle activity during maximum voluntary clench in different research diagnostic criteria for temporomandibular disorders (RDC/TMD) groups. Man Ther. 2008; 13: 437-44.
28. Bérzin F, Sakai E. Fundamentos da eletromiografia (EMG) – da teoria à prática. In: Sakai E., Fiuza SC, Martins NS, Dominguez-Rodrigues GC, Grimberg J, Pereira CB et al., organizators. Nova visão em ortodontia e ortopedia funcional dos maxilares. São Paulo: Santos; 2004. p.311-30.
29. Ferrel WR, Tennant N, Sturrock RD, Ashton L, Creed G, Brydson G et al. Amelioration of symptoms by enhancement of proprioception in patients with joint hypermobility syndrome. Arthritis Rheum. 2004; 20: 3323-8.
30. Rodrigues D, Siriani AO, Bérzin F. Effect of conventional TENS on pain and electromyographic activity of masticatory muscles in TMD patients. Braz Oral Res. 2004; 18: 290-5.
 Correspondence:
Correspondence:
Fernanda Pasinato
Julio de Castilhos Street, number 2905, apt 102,
Downtown, Uruguaiana city, Rio Grande do Sul,
Brazil, CEP: 97510-311
Phone: (55) 81179089; (55) 81 34010225
E-mail: fepas.fisio@yahoo.com.br
Received for publication: April 06, 2011
Accepted: June 17, 2011