Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.10 no.3 Piracicaba Jul./Set. 2011
ORIGINAL ARTICLE
Ultrastructure of buffalo tooth enamel: a possible replacement for human teeth in laboratory research
Luana de Nazaré Silva SantanaI; Mayara Sabrina LuzI; Nayara Cristina Monteiro CarneiroII; Aline Marques DiasII; Marcia Cristina dos Santos GuerraIII; Rafael Rodrigues LimaIII
I Master student in Animal Science, Federal University of Pará, Belém, Pará, Brazil
II Undergraduate student in Dentistry, Federal University of Pará, Belém, Pará, Brazil
III Institute of Biological Sciences, Federal University of Pará, Belém, Pará, Brazil
ABSTRACT
Buffalo production takes place in several areas worldwide. In Brazil, buffalo are raised mainly in the Northern region, specifically in the Marajó archipelago, where most of the herd is slaughtered for meat. This makes possible the extraction of numerous healthy teeth from these animals as replacements for human teeth in laboratory tests. Aim: To evaluate the morphology of enamel from species Bubalus bubalis as a replacement for human enamel in laboratory research studies, considering its wider availability in the Amazon region. Methods: After removal, the teeth were prepared for scanning electron microscopy (SEM). Teeth were sectioned in different planes – some were subjected to abrasion and others were merely polished for observation of surface enamel. All samples were submitted to a cleaning process, dried, sputter-coated with a platinum alloy and set for observation under SEM. Results: The SEM micrographs revealed an aprismatic surface enamel as well as prismatic enamel, the latter being similar to human enamel, in both arrangement and morphology. Conclusions: Buffalo enamel showed prismatic morphology, requiring further tests to corroborate its use as a substitute for human teeth.
Keywords: enamel, buffalo, SEM.
Introduction
Buffalo production began in Brazil during the late 19th century, particularly in the Marajó archipelago, located in the Northern region of the country. Recent estimates show that Brazil has the largest Bubalus bubalis herd in the Americas1. The large-scale buffalo production in that area is directed mainly towards meat production, resulting in high availability of biological material for other uses, including scientific studies.
Currently, laboratory dental research studies are limited by the small number of healthy extracted human teeth available, as well as by the ethical aspects in obtaining them. This has led to an increase in the illegal use of human teeth in research, through postmortem extraction and illegal trade in dental organs, which goes against Law 9434, from February 4th, 19972. As an alternative to these limitations, studies have been proposed using different animals, including several mammal species, to be adopted as experimental models3-5.
Several investigations have been carried out using teeth from different animals, such as bovines4-8, swine4,7,9, equines10-11 and others. Among these, bovine teeth have been most commonly used, due to easy acquisition and to the fact of having several morphological aspects similar to human teeth12-13.
The dental arch of a nine-month old buffalo already has 8 erupted permanent incisors and 12 premolars; adult animals have 32, with more 12 molars in the dentition1,14. Due to the need for an adequate substitute for human teeth in laboratory studies, buffalo teeth can be regarded as an interesting alternative animal model. Given the difficulty in using human teeth in scientific research studies, due both to access factors and ethical issues, an animal substitute as similar as possible to human teeth becomes extremely important15. Therefore, the objective of this study was to evaluate the morphology of tooth enamel from buffalo species Bubalus bubalis as a replacement for human enamel in laboratory studies, using scanning electron microscopy (SEM).
Material and methods
This investigation began by submitting the project to the Ethics Committee for Animal Experiment Research of the Federal University Pará (CEPAE- UFPA) and granting its approval under the protocol #BIO013/09.
Biological samples were obtained from 8 male adult buffaloes (Bubalus bubalis) from Marajó Archipelago. All samples were obtained from animals slaughtered for commercial purposes. Maxillary incisors were extracted, crowns removed from the roots, and organic residues adhered to the crown surfaces were mechanically removed using a soft-bristle toothbrush, preserving tissue integrity. Next, specimens were sectioned in different planes using a double-sided diamond disk set in a low-speed motor, in order to obtain enamel samples of various depths and section planes. After sectioning, the samples used to visualize surface enamel were polished with 04-μm-grain diamond paste to obtain a smoother surface. The samples selected for enamel observation in deeper planes were submitted to progressive abrasion using 1200-, 1500- and 2000-grit abrasive paper, after sectioning. Sections were immersed in ultrasonic bath with distilled water for 30 s. Next, the samples were kept for five min in a sodium hypochlorite solution at 1% in order to remove any remaining organic material, and returned to ultrasonic bath in distilled water for 30 s. They were immersed in HCl solution at 10% for 10 s in order to remove the smear layer resulting from the cutting process. For final detritus removal, the specimens were subjected to immersion in ultrasonic bath with distilled water for 60 s. The next process consisted of dehydrating the specimens in increasing concentrations of alcohol (70%, 90% and 100%), for 5 min in each concentration. The samples were then dried at room temperature, set and sputter-coated with a platinum alloy. SEM micrographs of buffalo enamel were obtained for the different regions mentioned, using a scanninglelectron microscope (LEO-1430; Carl Zeiss, Oberkochen, Germany) under different magnifications.
Results
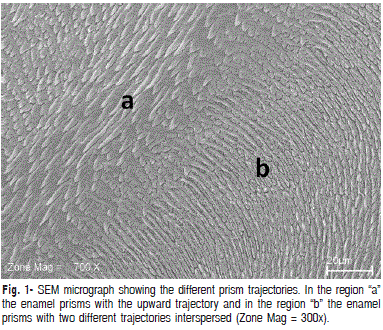
The ultrastructure pattern found in buffalo enamel revealed several morphological aspects similar to those found in human enamel. As seen in Figure 1, obtained from deep enamel planes, prisms were observed arranged in different directions – perpendicular and parallel to the plane in which they are viewed. This aspect is similar to the prismatic pattern of human teeth, which follow an irregular course.
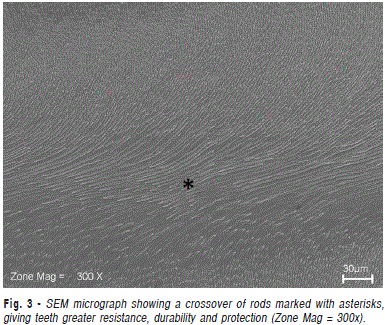
In Figure 2, under 700x magnification obtained from deep planes, the final portion of the long axis of the rods can be seen in a region where prisms are all arranged in the same direction. Figure 3 shows a crossover of rods arranged in rows with alternate distributions. This complex organization gives greater resistance, durability and protection to teeth, and is also commonly found in human tooth enamel16-17.

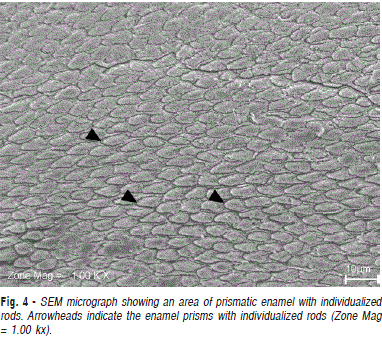
Observing the surface structure (Figure 4), prismatic enamel is evidenced in individualized rods, showing their diameters. In Figure 5, also from the surface plane, this individual pattern is lost, suggesting the presence of aprismatic enamel. Another relevant characteristic observed in Figures 4 and 5 is the existence of a mineralized tissue that circles the enamel rods, an interrod enamel sheath. From the visualization of this histological finding, the existence of interprismatic enamel in buffalo dental tissue is proposed. These aspects approximate the ultrastructural pattern of both.
Discussion
Teeth featuring microscopic morphology similar to recent vertebrates appeared approximately 460 million years ago. Some Agnathan fish species developed surface structures named odontoids, which were initially located outside the oral cavity. Odontoids consisted of tissues similar to those found in current vertebrates – pulp chamber, dentin, covered by hypermineralized enameloid material. The evolution of these structures is their displacement towards the inner oral cavity led to the emergence of dental elements similar to those known today. Feeding habits and ecological adaptations directly influenced the acquisition of different anatomic shapes, represented by incisors, canines, premolars and molars, as well as structural modifications in dental tissues15.

Enamel underwent evolution processes resulting in a prismatic pattern, currently found in higher mammals. It is known that human tooth enamel is a complexity arranged hypermineralized tissue, secreted by ameloblasts (cells of ectodermic origin). Its extracellular matrix consists of approximately 96% mineral material and 4% organic matter and water; inorganic content is formed basically by hydroxyapatite crystals16.
The basic structural units of enamel are prisms and interprismatic substance. Prisms are rod-shaped structures formed basically by ordered and densely arranged hydroxyapatite crystals. These rods, involved in interrod enamel, represent most of the thickness of dental tissue – prismatic enamel. Internally (the deepest layer, next to dentin) and externally (superficially) to it, there are thin layers of aprismatic enamel, without rods16,17.
A microscopic morphology similar to that found in this investigation has been described in teeth from other mammals. According to Lopes et al.9 (2006), teeth from monkeys, dogs and swine show an enamel mineralization pattern similar to humans. Furthermore, other authors, such as Fejerskov18 (1979), Limeback et al.19 (1992) and Popowics, Rensberger and Herring20 (2001) studied the dental tissues of these mammals and found several similarities to humans, such as size, macro- and microscopic morphology, and development period.
Bovine teeth have shown results akin to human teeth in laboratory tests21-22. Schilke et al.23 (1998) performed a study on enamel morphology and did not find differences hardness, which was similar to human enamel. Moreover, the ratio of organic and inorganic components is similar in both tissues. Oesterle et al.12 (1998) did not observe differences in the adhesion of materials to human or bovine enamel, and attributed these findings to the similar microstructure of both substrates. However, some authors reported small differences in the behavior of human and bovine enamel in certain laboratory tests24. This demonstrates the need to continue researching new animal models, as done in the present study.
Buffaloes emerge as a promising species in scientific studies. This study showed that the ultrastructural morphology of buffalo enamel was similar to that of human enamel, suggesting that it may be an alternative to human teeth in enamel studies. However, further studies are needed to evaluate the behavior of buffalo enamel in tests of adhesion to restorative materials, hardness evaluation, analysis of radiographic aspects, as well as more detailed investigations of its mineral composition.
References
1. Santos FCF, Sousa AL, Machado Júnior AAN, Lima FC, Ribeiro F. Análise morfológica dos dentes incisivos de búfalos e sua relação com a idade de abate. Cienc Animal Bras. 2008; 9: 506-11. [ Links ]
2. Brasil. Lei n° 9.434. Dispõe sobre a remoção de órgãos, tecidos e partes do corpo humano para fins de transplante e tratamento e dá outras providências. Diário Oficial da União. 1997.
3. Weinberg MA, Bral M. Laboratory animal models in periodontology. J Clin Periodontol. 1999; 26: 335-40.
4. Fonseca RB, Haiter Neto F, Fernandes Neto AJ, Barbosa GAS, Soares CJ. Radiodensity of enamel and dentin of human, bovine and swine teeth. Arch Oral Biol. 2004; 49: 919-22.
5. Camargo CHR, Sivieiro M, Camargo SEA, Oliveira SHG, Carvalho CAT, Valera MC. Topographical, diametral and quantitative analysis of dentin tubules in the root canals of human and bovine teeth. J Endod. 2007; 33: 422-6.
6. Resende AMR, Gonçalves SEP. Avaliação da infiltração marginal em dentes humanos e bovinos com dois diferentes sistemas adesivos. Cienc Odontol Bras. 2002; 5: 38-45.
7. Abuabara A, Santos AJS, Aguiar FHB, Lovadino JR. Evaluation of microleakage in human, bovine and swine enamels. Braz Oral Res. 2004; 18: 312-6.
8. Fonseca RB, Haiter Neto F, Carlo HL, Soares CJ, Sinhoreti MAC, Puppin-Rotani RM et al. Radiodensity and hardness of enamel and dentin of human and bovine teeth, varying bovine teeth age. Arch Oral Biol. 2008; 53: 1023-9.
9. Lopes FM, Markarian RA, Sendyk CL, Duartz CP, Arana-Chavez VE. Swine teeth as potential substitutes for in vitro studies in tooth adhesion: A SEM observation. Arch Oral Biol. 2006; 51: 548-51.
10. Muylle S, Simoens P, Lauwers H. The dentinal structure of equine incisors: a light and scanning electron-microscopic study. Cells Tissues Organs. 2000; 167: 273-84.
11. Muylle S, Simoens P, Lauwers H. The distribution of intratubular dentine in equine incisors: a scanning electron microscopic study. Equine Vet J. 2001; 33: 65-9.
12. Oesterle L, Shellhart W, Belanger G. The use of bovine enamel in bonding studies. Am J Orthod Dentofacial Orthop. 1998; 114: 514-9.
13. Posada MC, Sanches CF, Gallego GJ, Vargas AP, Restrepo LF, López JF. Dientes de bovino como sustituto de dientes humanos para su uso em la odontologia. Rev CES Odontol. 2006; 19: 63-8.
14. Seixas VNC, Cardoso EC, Araújo CV, Pereira WLA, Viana RB. Determinação da cronologia dentária de machos bubalinos (Bubalus bubalis) criados no estado do Pará. Cienc Animal Bras. 2007; 8: 529-35.
15. Koussoulakou DS, Margaritis LH, Koussoulakos SL. A Curriculum Vitae of teeth: Evolution, generation, regeneration. Int J Biol Sci. 2009; 5: 226- 43.
16. Nanci A. Ten Cate, Histologia oral: desenvolvimento, estrutura e função. Rio de Janeiro: Elsevier; 2008.
17. Durso G, Abal A. Variabilidad de la morfología de los prismas del esmalte dental humano. Acta Microscopica. 2008; 17: 1-8. 18. Fejerskov O. Human dentition and experimental animals. J Dent Res. 1979; 58: 725-34.
19. Limeback H, Schlumbohm C, Sen A, Nikiforuk G. The effects of hypocalcemia/hypophosphatemia on porcine bone and dental hard tissues in an inherited form of type 1 pseudo-Vitamin D deficiency rickets. J Dent Res. 1992; 71: 346-52.
20. Popowics TE, Rensberger JM, Herring SW. The fracture behavior of human and pig molar cusps. Arch Oral Biol. 2001; 46: 1-12.
21. Muench A, Da Silva E.M, Ballester RY. Influence of different dentinal substrates on the tensile bond strength of three adhesive systems. J Adhesive Dent. 2000; 2: 209-12.
22. Moreira DM, Almeida JF, Ferraz CC, Gomes BP, Line SR, Zaia AA. Structural analysis of bovine root dentin after use of different endodontics auxiliary chemical substances. J Endod. 2009; 35: 1023-7.
23. Schilke R, Bauss O, Lisson JA, Schuckar M, Geurtsen W. Bovine dentin as a substitute for human dentin in shear bond strength measurements. Am J Orthod Dentofacial Orthop. 1998; 114: 514-9.
24. Nakamichi I, Iwaku M, Fusayama T. Bovine teeth as possible substitutes in the adhesion test. J Dent Res. 1983; 62: 1076-81
 Correspondence:
Correspondence:
Rafael Rodrigues Lima
Instituto de Ciências Biológicas
Laboratório de Neuroproteção e Neurorregeneração Experimental. Universidade Federal do Pará
Rua Augusto Corrêa, 1. Campus do Guamá
CEP: 66075-900. Belém - Pará - Brazil
Phone/Fax:(55) 91 3201 7891
E-mail: rafalima@ufpa.br
Received for publication: January 31, 2011
Accepted: april 29, 2011













