Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.10 no.3 Piracicaba Jul./Set. 2011
ORIGINAL ARTICLE
Microhardness of nanofilled composite resin light-cured by LED or QTH units with different times
Ana Isabelle Salvador GroningerI;Giulliana Panfiglio SoaresII; Robson Tetsuo SasakiIII; Glaucia Maria Bovi AmbrosanoIV José Roberto LovadinoV; Flávio Henrique Baggio AguiarVI
I Undergraduate student, Department of Restorative Dentistry, Piracicaba Dental School, University of Campinas, Brazil
II DDS, MS, PhD student, Department of Restorative Dentistry, Piracicaba Dental School, University of Campinas, Brazil
III DDS, MS student, Department of Restorative Dentistry, Piracicaba Dental School, University of Campinas, Brazil
IV DDS, MS, PhD, Assistant Professor, Department of Community Dentistry and Statistics, Piracicaba Dental School, University of Campinas, Brazil
V DDS, MS, PhD, Chairman, Department of Restorative Dentistry, Piracicaba Dental School, University of Campinas, Brazil
VI DDS, MS, PhD, Assistant Professor, Department of Restorative Dentistry, Piracicaba Dental School, University of Campinas, Brazil
ABSTRACT
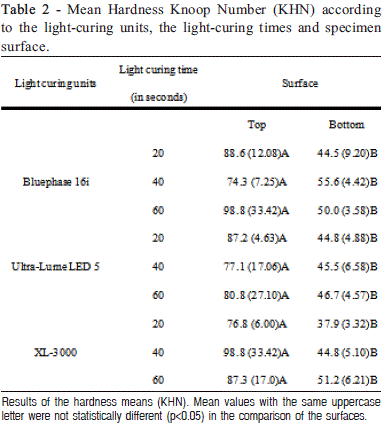
Aim: To evaluate the influence of light-curing units and light-curing time on the microhardness of a nanofilled composite resin. Methods: Forty-five composite resin (Z350 - 3M) specimens were randomly prepared using Teflon ring molds (4.0 mm internal diameter and 2 mm depth) and divided into nine experimental groups (n=5): three polymerization units (conventional - 450 mW/ cm2; 2nd generation LED - 1100 mW/cm2; and 3rd generation LED - 700 mW/cm2) and three lightcuring times (20 s, 40 s, and 60 s). All specimens were polymerized with the light-curing tip positioned 8 mm far from the top surface of the specimen. After 24 h, Knoop microhardness measurements were made on the top and bottom surfaces of the specimen, with a load of 10 g for 10 s. Five indentations were made on each surface. All results were analyzed statistically by subdivided parcel ANOVA (Split-Plot) and Tukey's tests (p<0.05). Results: There were no statistically significant differences for the polymerization unit and light-curing time factors in either top or bottom surface. For all experimental conditions, the top surfaces showed greater hardness than the bottom surfaces (p<0.0001). Conclusions: The mode of polymerization and the lightcuring time did not affect the hardness of the nanofilled composite resin, and increasing the lightcuring time did not improve the hardness of the bottom surface of the composite resin.
Keywords: composite resins, hardness.
Introduction
Composite resin has been described as an esthetic restorative material with excellent physical and mechanical properties1 when adequate polymerization is obtained2. However, many variables affect the amount of light energy received on the top and bottom surfaces of a composite resin restoration, such as the design and size of the light guide, distance of the light guide tip from the composite resin, power density, exposure duration, shade and opacity of the composite resin, increment thickness, and material composition3-5. If the restoration does not receive sufficient total energy, various problems may arise, e.g., reduced degree of conversion, increased cytotoxicity, reduced hardness, increased pigmentation, decreased dynamic elasticity modulus, increased wear, increased marginal leakage and weak a bond among the tooth, adhesive, and restoration3-4,6.
Clinically, deficient polymerization can occur in deeper cavities due to the dispersion of light energy that occurs because of the distance between the light-curing tip and the first composite resin increment7. In a deeper cavity, the interface between the composite resin and the tooth structure may be less polymerized, and exposure of this interface to the oral environment can generate marginal discolorations, restoration fractures, and composite resin and adhesive solubility, leading to microleakage and secondary caries7. However, few studies have been conducted with the purpose of testing the depth of composite resin curing in situations where the light-curing tip is distant from the filling material, as in the aforementioned clinical situations. Thus, it is important to evaluate the minimum light-curing time required for correct polymerization in accordance with the light-curing unit used. The aim of this in vitro study was to evaluate the influence of the light-curing time using LED or QTH on the hardness of the top and bottom composite resin surfaces in a clinical simulation when the light-curing tip was at distance of 8 mm and the material thickness was 2 mm.
Material and methods
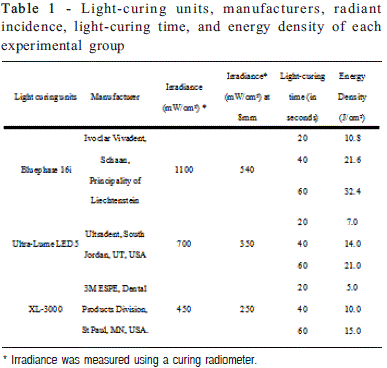
A nanofilled composite resin, Z350 (3M-ESPE Dental Products, St. Paul, MN, USA), was used in this study. Fortyfive cylindrical specimens were prepared using Teflon ring molds (4.0 mm internal diameter and 2 mm depth) held between two glass slabs separated by Mylar matrix strips and then pressed with a 500 g static load. The cavities were randomly filled in one resin increment and polymerized according to the nine experimental groups (n=5): three polymerization units (conventional - 450 mW/cm2; 2nd generation LED - 1100mW/cm2; 3rd generation LED - 700mW/cm2) and three light-curing times (manufacturer's recommended time - 20s; twice the manufacturer's recommended time - 40s; and thrice the manufacturer's recommended time - 60s). Polymerization was performed with a source-to-specimen distance of 8 mm, checked with a digital caliper (Mitutoyo, USA). Irradiance of the light curing units was measured using a curing radiometer (Demetron Research Corp., Danbury, CT, USA). The energy density was calculated according to the product of the irradiance of the light curing unit (mW/cm2) X exposure duration (s) (Table 1).
Each specimen was removed from the mold and stored in a lightproof container at 37C and 95 ± 5% relative humidity for 24 h. After this period, the specimens were washed and the Knoop hardness on the bottom and top surfaces was tested using a Knoop hardness indenter (FM - Future Tech Corp., Japan) under a 10 g load for 10 s. Five measurements were made at the approximate center of the specimen4. The values, obtained in micrometers, were converted to Knoop Hardness Number (KHN) using computer software (Microsoft Excel for Windows®).
The Knoop hardness values obtained on top and bottom surfaces were subjected to the subdivided parcel ANOVA (Split-Plot) test (p=0.05) and Tukey's test at the 5% significance level. The light-curing unit and light-curing time factors were considered in the parcels, and the factor surface (top and bottom surfaces) was considered in the sub-factor.
Clinical Examination
Clinical examination was performed according to the criteria proposed by Newton (1962) for DS classification in types I, II, III11. Type I showed localized inflammation or pinpoint hyperemia; type II showed a generalized erythema and type III comprised papillary hyperplasia of the palate. All patients who met these criteria were included in the test group.
Denture evaluation was done by direct examination and the patients were questioned about their age, gender, smoking and alcohol use, systemic health status, use of medicines (class, dose and frequency), oral health and denture wearing. The criteria to establish smoking and alcohol habits were the following: smoker was the individual who had the habit of daily use of tobacco at the time of enrolment in the study and alcohol habit was the use of distilled and/or fermented beverage two or more times a week at the time of enrolment in the study.
Samples and Techniques
WUS was collected during 3 min in a sterile tube, under standard conditions: between 8 AM and 11 AM, no feeding, drinking, smoking or hygienic habits allowed for 60 min before the test section.
After this step, a sterile swab was inserted in the tube and was direct sampled in half of a CC Plate (PlastLabor, Rio de Janeiro, RJ, Brazil). On the other half of the plate, an oral swab from palatal mucosa were taken by passing this sterile cotton several times across the mucosal surface and then seeding onto the CC plate.
Exfoliative cytology was performed following the swabbing. The technique consisted in passing a Cytobrush several times across the palate surface and disposed the collected material on two glass slides and fixed in absolute alcohol. These slides were sent to the Oral Pathology Department and stained by Periodic Acid Schiff (PAS) and Papanicolaou techniques. The fungal presence was searched by analyzing the presence of hyphal outgrowths (abundant, moderate, scarce and absent).
After 48 h of incubation at 37ºC, the CC plates were photographed and the digitized images were entered into the ImageJ software (ImageJ 1.44f, Wayne Rasband, Bethesda, MD, USA) for analysis of colonies. The presumptive identification of Candida species was based on the criteria proposed by Odds and Bernaerts (1994), who described the species by the color of the colony. C. albicans colonies are described by their green color, C. tropicalis colonies based on their dark-blue to blue-gray color, surrounded by a dark/ pink halo, C. glabrata by their white/dark pink/purple range of colors and C. krusei colonies based on their pale pink color and downy/rough appearance with pale edges. The colonies presenting other colors were classified as Candida ssp.12. The identification of the colors of the colonies in the digitalized images was made by two calibrated dentists.
Patients who had moderate and abundant proportions of hyphal outgrowths in the cytological exams, along with positive clinical signs and positive culture tests, were considered as having candidiasis.
The statistical tests were made in the DS group only, with comparison between single and mixed colonization when considering the tobacco and alcohol habits and use of systemic drugs, by the Fisher's exact test.
Results
The patients with DS had mean age of 58.1 years (SD 11.6) being 28 females and 10 males. In the control group, the mean age was 62.1 years (SD 6.9), with 18 females and 2 males. Direct swabbing from the WUS and palatal mucosa revealed colonization in 37 (97.3%) of patients with DS. In the control group, 11 (55.0%) patients presented colonization from WUS, and swabbing of palatal mucosa was positive to reveal colonization in 6 (30.0%) patients.
In the DS group, 22 patients were using medicines and the major group of drugs taken was osmotic diuretics (10 patients). In the control group, 13 patients were using medicines and angiotensin-converting enzyme (ACE) inhibitors were the most frequently taken drugs, used by 8 patients.
Direct examination of the denture prosthesis revealed the majority of them with some inadequacy in shape, contour and surface as well as inadequate hygiene. The mean time of denture wearing was 11.6 years (SD 12.3).
The isolates showed C. albicans as the most prevalent between both groups, respectively in 89.4% from the patients with DS and 40.0% from the control group. About the nonalbicans species in the DS group, the most encountered were C. krusei (31.5%), followed by C. glabrata (21.0%), C. tropicalis (15.7%) and Candida spp (2.6%). In the control group, the non-albicans species encountered were C. glabrata (23.0%) and C. tropicalis (23.0%). To obtain the prevalence of colonization, when a patient harbored two or more species, these were counted as separate events, i.e., if a patient had colonization by C. albicans and C. krusei, they were counted as two species.
The WUS swabbing from DS patients revealed mixed colonization in 20 of them, C. albicans only in 12, nonalbicans in 3 and no colonization in 3 patients. The swabbing of palatal mucosa revealed mixed colonization from 15 patients, 17 by C. albicans only, 3 of non-albicans and 3 with no colonization.
Cytology showed positive results only in 8 (22.2%) patients from the DS group, with 4 in the moderate range and 4 in the scarce range. In the control group, only 1 patient (7.6%) presented positivity to the test, in the scarce range. All patients with positive exfoliative cytology results were also positive for CC.
Regarding the presumptive identification, the data were distributed with all patients allocated as shown in Tables 1 and 2. The patient was considered as harboring mixed yeast population when presented mixed colonization by swabbing of WUS and/or palatal mucosa. When the patients were allocated in different groups, smokers revealed an increased proportion in mixed colonization, with a significant difference between the patients with and without the tobacco habit (p=0.0051).
Four patients with positivity to the tests and clinical indication of therapeutics received treatment for candidiasis with nystatin topical and all individuals were sent to receive new dentures.
Discussion
DS is a frequent finding among denture wearers. Although its etiology is unknown, the influence of the colonization by Candida spp. has already been described. At the same time, not all patients with DS present colonization by Candida spp., although some of them were not classified even as carriers. In the present study, only 1 patient with DS showed absence of colonization.
Abaci et al. (2010), demonstrated that the number of yeast cells in the saliva of patients with DS was <400 CFU/ mL in 11 individuals and >400 CFU/mL in 19 individuals. In addition, all patients with DS, who were complete denture and removable partial denture users, presented colonization by Candida spp. from saliva samples. However, one had no colonization from swabs taken from the palatal mucosa13.
No patient, in either the DS and or control group, complained about symptoms like continuous burning sensation or taste disorder. Although these symptoms are relatively common in patients with DS, it has been reported that there is no association between burning mouth syndrome and prevalence of Candida spp.14. In the same way, an increase in oral yeasts is not necessarily associated with changes in mouth sensation alone15.
The direct seeding without saliva dilution or any other procedure for sampling is preferable for clinical use, as performed in our study, bringing advantages such spending less time to carry out the technique and achievement of satisfactory presumptive results that can guide the choice of therapeutic support. The time elapsed for presumptive identification with CC, as used in the present study, was about 48 h. The identification of yeasts in a mycology laboratory with conventional media is time-consuming and cumbersome as it requires between 4 and 6 days16.
Cytology showed positive results in 22.2% of the cases in the DS group, demonstrating the inefficiency of this method for the diagnosis of oral candidosis when the material is obtained from scraping and/or swabbing. Although the method is valuable to distinguish yeasts from hyphal forms, it is less sensitive than culture methods17, as seen in the present study.
Colonization by Candida spp. in both groups represented the importance of the denture use on oral yeast carriage. Old age, clinical signs of oral dryness, denture wearing and reduction in whole unstimulated salivary flow increase the prevalence of oral yeasts15. Also, if the balance of the normal flora is disrupted or the patient's immune defenses are compromised, Candida spp. can invade mucosal surfaces and cause disease manifestations18. In addition, the high incidence of colonization in the patients with DS (97.3%) corroborates with the long-term period of the denture use (11 years on the average). This may have positively influenced the colonization and predisposed to the installation of DS.
Regarding the mixed yeast populations from patients with DS, non-albicans species have been found in only 14.7% individuals in a previous investigating Candida spp. incidence in denture wearers13. In the present study, the frequency of colonization by non-albicans and C. albicans plus non-albicans was higher. These findings suggest that our patients presented more mixed fungal populations because their clinical and/or systemic conditions might have predisposed them to the Candida infection.
In addition, when the patients with DS were evaluated by groups, which means separation of the patients according to their specific conditions, smokers presented 90% of mixed colonization and no C. albicans colonization alone. A recent study19 found a frequency of 54.8% of C. albicans and 45.2% of non-albicans species in healthy smokers, demonstrating a possible influence of smoking habit on colonization, although nonsmokers presented 35.1% of non-albicans colonization. In addition, they concluded that there was a marginally significant positive correlation between the number of cigarettes smoked per day and the density of candidal growth from oral rinse cultures. Figueiral et al. 20 (2007) indentified C. albicans, C. glabrata and C. tropicalis in a group of patients with DS, and C. tropicalis appeared always together with one of the other species. Those authors concluded that yeasts, particularly C. albicans, are associated with DS. In the present study, only 2 patients in the control group were smokers and neither of them presented colonization of C. albicans only.
Our results revealed a higher colonization in patients using diuretics, which could explain the reduced salivary flow, favoring the presence of yeasts. However, it is important to emphasize that there were no dryness complains. Torres et al.21 reported a frequency of 28% of mixed colonization in patients with xerostomia, being 88.4% users of medications and 58% of these patients diagnosed with hyposalivation.
The data obtained in the present study and reported in the literature revealed the influence of some variable conditions on yeast carriage and the presence of infection. When we separated the patients into different groups, we intended to evaluate the influence of each variable in colonization, aiming at determining whether some clinical aspects and habits could result in a difference between groups in an important rate. Smoking showed a high difference when the patients were allocated as smokers and non-smokers, with rates of 90% of mixed colonization.
It is worth mentioning that our study used the presumptive identification, but our objective was focused on sampling techniques. CC can be extremely helpful in a clinical stomatology service routine, facilitating the detection of mixed cultures of yeasts and allowing direct identification of C. albicans, as well as rapid presumptive identification of other species like C. glabrata, C. tropicalis, Candida krusei and even Sacharomyces cerevisiae22. However, Tintelnot et al.23 (2000) described CC as an insufficiently selective medium for isolation of C. dubliniensis as it can produce dark green in primary cultures. Our findings also reinforces the need to employ other common tests, such as germ tube observation and carbohydrate assimilation, to institute an appropriate therapy for cases of undiagnosed Candida spp., recurrent infections or lack of response to antifungal drugs.
It has been reported that there is increasing evidence that more than one Candida spp. may simultaneously colonize mucosal habitat. The need to investigate the factors that play a role in the initial attachment and subsequent colonization of denture-base materials and the oral mucosa of patients subjected to Candida infection should be known, controlled and may prevent the disease24. Differentiation between species is also important for the identification of infection. CC was used as an easy and rapid technique, reaching the target to an appropriated diagnosis and institution of adequate therapy. The findings of the present study also revealed the prevalence of infections by C. albicans followed by C. krusei and C. glabrata. In an extensive review, Samaranayake and Samaranayake25 (2001) reported that C. glabrata emerges as the second most prevalent species, frequently isolated from acrylic denture surface and the palatal mucosa. Our results led us to encourage the use of CC in stomatology daily practice. It enables better knowledge of the most prevailing species of the genus Candida in the oral mucosa, resulting in a better management and control of infections, and, even for screening purposes, with faster and less expensive results.
Finally, it is important to diagnose patients with DS, recommending the adequate treatment for each case. Even asymptomatic patients should be oriented and treated if necessary, due to the importance of the oral mucosa as a primary source of reinfection, mainly in the event of an eventual hospitalization, which would affect substantially their quality of life.
In conclusion, the use of CC was an effective subsidiary technique for the diagnosis of oral candidosis in patients with DS, with the additional advantage of enabling presumptive species identification within the genus. Smoking seemed to play a role in the colonization of oral mucosa by mixed C. albicans and non-albicans species. The results also points to a more prevalent mixed colonization in patients with DS.
References
1. Sweet SP. Selection and pathogenicity of Candida albicans in HIV infection. Oral Dis. 1997;3(Suppl 1):S88-95. [ Links ]
2. Farah CS, Ashman RB, Challacombe SJ. Oral candidosis. Clin Dermatol. 2000;18(5):553-62.
3. Odds FC. Candida infections: an overview. Crit Rev Microbiol. 1987;15(1):1-5.
4. Jorge Júnior J, de Almeida OP, Bozzo L, Scully C, Graner E. Oral mucosal health and disease in institutionalized elderly in Brazil. Community Dent Oral Epidemiol. 1991;19(3):173-5.
5. Budtz-Jorgensen E. Ecology of Candida-associated denture stomatitis. Microb Ecol Health Dis. 2000;12:170-85.
6. Silva GM, Silveira FRX, Pires, MFC. Adherence to HeLa cells, typing by killer toxins and susceptibility to antifungal agents of Candida dubliniensis strains. Braz Oral Res. 2007; 21: 87-91.
7. Schelenz S, Abdallah S, Gray G, Stubbings H, Gow I, Baker P et al. Epidemiology of oral yeast colonization and infection in patients with hematological malignancies, head neck and solid tumors. J Oral Pathol Med. 2011; 40: 83-9.
8. Rautemaa R, Rusanen P, Richardson M, Meurman JH. Optimal sampling site for mucosal candidosis in oral cancer patients is the labial sulcus. J Med Microbiol. 2006; 55: 1447-51.
9. Sendid B, François N, Standaert A, Dehecq E, Zerimech F, Camus D et al. Prospective evaluation of the new chromogenic medium CandiSelect 4 for differentiation and presumptive identification of the major pathogenic Candida species. J Med Microbiol. 2007; 56: 495-9.
10. Bernal S, Martín Mazuelos E, García M, Aller AI, Martínez MA, Gutiérrez MJ. Evaluation of CHROMagar Candida medium for the isolation and presumptive identification of species of Candida of clinical importance. Diagn Microbiol Infect Dis. 1996; 24: 201-4.
11. Newton AV. Denture sore mouth: a possible aetiology. Br Dent J. 1962: 112-357.
12. Odds FC, Bernaerts R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994; 32: 1923-9. 13. Abaci O, Haliki-Uztan A, Ozturk B, Toksavul S, Ulusoy M, Boyacioglu H. Determining Candida spp. incidence in denture wearers. Mycopathologia. 2010; 169: 365-72.
14. Cavalcanti DR, Birman EG, Migliari DA, Silveira FRX. Burning mouth syndrome: clinical profile of Brazilian patients and oral carriage of Candida species. Braz Dent J. 2007; 18: 341-5.
15. Shimizu C, Kuriyama T, Williams DW, Karasawa T, Inoue K, Nakagawa K et al. Association of oral yeast carriage with specific host factors and altered mouth sensation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 105: 445-51. 16. Willinger B, Manafi M. Evaluation of CHROMagar Candida for rapid screening of clinical specimens for Candida species. Mycoses. 1999; 42: 61-5.
17. Williams DW, Lewis MA. Isolation and identification of Candida from the oral cavity. Oral Dis. 2000; 6: 3-11.
18. Mardegan RC, Foglio MA, Gonçalves RB, Höfling JF. Candida albicans proteinases. Braz J Oral Sci. 2006; 5: 944-52.
19. Darwazeh AM, Al-Dwairi ZN, Al-Zwairi AA. The relationship between tobacco smoking and oral colonization with Candida species. J Contemp Dent Pract. 2010; 11: 17-24.
20. Figueiral MH, Azul A, Pinto E, Fonseca PA, Branco FM, Scully C. Denture-related stomatitis: identification of aetiological and predisposing factors - a large cohort. J Oral Rehabil. 2007; 34: 448-55.
21. Torres SR, Peixoto CB, Caldas DM, Silva EB, Akiti T, Nucci M, et al. Relationship between salivary flow rates and Candida counts in subjects with xerostomia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 93: 149-54.
22. Bouchara JP, Declerck P, Cimon B, Planchenault C, de Gentile L, Chabasse D. Routine use of CHROMagar Candida medium for presumptive identification of Candida yeast species and detection of mixed fungal populations. Clin Microbiol Infect. 1996; 2: 202-8.
23. Tintelnot K, Haase G, Seibold M, Bergmann F, Staemmler M, Franz T, Naumann D. Evaluation of phenotypic markers for selection and identification of Candida dubliniensis. J Clin Microbiol. 2000; 38: 1599-608.
24. Pereira-Cenci T, Del Bel Cury AA, Crielaard W, Ten Cate JM. Development of Candida-associated denture stomatitis: new insights. J Appl Oral Sci. 2008; 16: 86-94.
25. Samaranayake YH, Samaranayake LP. Experimental oral candidiasis in animal models. Clin Microbiol Rev. 2001; 14: 398-429.
 Correspondence:
Correspondence:
Gustavo Davi Rabelo Faculdade de Odontologia da Universidade de São Paulo – Departamento de Estomatologia Av. Prof. Lineu Prestes, 2227 - São Paulo - SP CEP: 05508-000 Phone: 11-30917902
E-mail: vedovelloorto@terra.com.br
Received for publication: April 27, 2010
Accepted: July 13, 2011













