Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.10 no.3 Piracicaba Jul./Set. 2011
ORIGINAL ARTICLE
How the anterior, middle and posterior portions of the temporalis muscle work during mastication
Mirian NagaeI;Fausto BérzinII; Marcelo Corrêa AlvesIII; Maria da Graça Rodrigues BérzinIV
I Speech-language pathologist, Msc, PhD, Faculty of Medical Science Department, University of Campinas, Campinas, SP, Brazil
II DDS, MSD, PhD, Professor, Morphology Department, Piracicaba Dental School, University of Campinas, Brazil
III IT Analyst, Msc, PhD, Superior School of Agriculture Luiz de Queiroz, University of São Paulo, Brazil
IV Psychologist, Msc, PhD, Electromyography Center, Piracicaba, SP, Brazil
ABSTRACT
Aim: The aim of this study was to investigate mean electrical activity and how the anterior, middle, and posterior portions of the temporalis muscle work during mastication. Methods: The sample consisted of 16 healthy male college freshmen trichotomized, aged between 18 and 25 years, with Angle's Class I and no temporomandibular disorders. Electromyographic (EMG) recordings were made in anterior, middle and posterior portions of the temporalis muscle during mastication for 5 s. Results: It was found a significantly lower RMS value in the posterior portion (RMS: 1243.92) compared with those of the anterior (RMS: 2149.77) and middle (RMS: 2531.38) portions. Conclusions: There is an association between the portions of the temporalis muscle. It was found a significantly lower RMS value in the posterior portion showing that the anterior and middle portions of the muscle have a predominant function of maintaining movement during mastication.
Keywords: electromyography, mastication, temporal muscle.
Introduction
The temporalis muscle is capable of performing different functions in the stomatognathic system and can have an agonist, antagonist and synergistic action depending on the activity involved1. This muscle has a complex pennate architecture2-4 that allows wide movements and great range of ajustment5 for maintaining the stability of the mandible. It is divided into anterior, middle and posterior portions, which protrudes, elevates and retracts the mandible, respectively2.
The wide movements of the mandible produced during mastication allow studying the integrated actions of the different portions of the temporalis muscle. However, little is known on how the anterior, middle and posterior portions work together to produce movement3-4.
Most electromyography (EMG) studies have focused on maximum voluntary contraction (CVM), showing greater activity of the posterior portion of the temporalis muscle6. Mastication studies have reported inconsistent results showing similar actions of the portions4 activity only of the anterior and posterior portions7 and significant activity of the anterior and middle portions only3.
Several studies8-9 have reported the clinical importance of the temporalis muscle for mandibular stability and its high susceptibility in patients with morphological deviations10 such as malocclusion and intermaxillary disproportion11-13.
EMG findings have been widely applied in dental practice14 for diagnosis, orthodontic care planning and assessment of devices such as occlusal splints2. However, it is not yet clear how the portions of the temporalis muscle work together in healthy individuals. This knowledge can allow comparisons in individuals with dental and skeletal deviations. The present study aimed to investigate how the anterior, middle, and posterior portions of the temporalis muscle work during mastication and to assess mean electrical activity of each portion in Angle's Class I individuals with the use of surface EMG. The study hypothesis is that there is an association between the muscle portions and that mean electrical activity decreases anteroposteriorly.
Material and methods
The study was approved by the Ethics Committee of the School of Medical Sciences of the University of Campinas (UNICAMP) (protocol # 315/2011).
The sample consisted of 16 healthy male college freshmen trichotomized, aged between 18 and 25 years, with Angle's Class I and no temporomandibular disorders.
EMG recordings were performed using Myosystem BR1, Myosystem software version 2.52 (DataHominis Tecnologia Ltda.) with signal conditioning, 12-bit resolution, 112 dB and 60 Hz common mode rejection ratio (CMRR) and A/D Myosystem converter (model PCI-DAS 1200, Prosecon Ltda.).
The reference electrode was positioned on the manubrium of the sternum bone of a volunteer. Signals were captured using disposable passive bipolar surface Ag/AgCl electrodes (Noraxon USA Inc., model 272), of 1 cm diameter and positioned with a fixed 1-cm interelectrode distance, connected to a preamplifier (Lynx Tecnologia Eletrônica Ltda., model PA 1010-VA) with gain of 20 times forming a differential circuit.
In the temporalis muscle, anterior electrodes were positioned 1 cm above the anterior portion of the zygomatic arch and the upper margin of the coronoid process near the zygomatic-temporal suture and lateral margin of the supraorbital ridge, and vertically arranged slightly forward15.
In the muscle's middle portion, electrodes were positioned slightly oblique at a 2 cm distance from the external ear canal16. Posterior electrodes were positioned about 1 cm away from the middle portion of the temporalis muscle and arranged at a 15-degree angle in the Frankfurt horizontal plan6. A test of muscle function17 was performed after electrodes had been attached to ensure its adequate positioning.
All EMG recordings were made during regular mastication for 5 s. The mastication cycles were during normal right and left chewing. As it was not known which side was determinant for the differences, the right side was chosen at random. The sample capture for signal was of 2 kHz. All subjects were given a chewing gum to assess mastication.
EMG recordings obtained were band pass filtered at 20-500 Hz using a Butterworth filter. The mean electrical activity of the cycles was estimated using the Root Mean Square18 (RMS) approach. Analysis of variance was performed to compare mean RMS values of the three portions of the temporalis muscle. Tukey's Studentized range (HSD) test for significance (p < 0.05) was used for simultaneous multiple comparisons. Since data from the three muscle portions were collected from the same individual, repeated measures analysis was performed using SAS GLIMMIX procedure as it allows to adjust data from populations with different distributions19 (SAS software; SAS Institute Inc., Cary, NC, USA).
Results
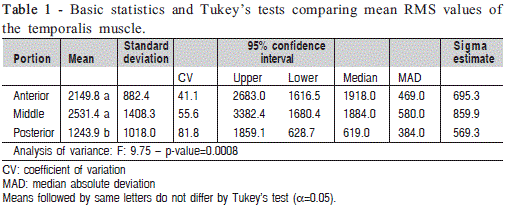
The F-test provides strong evidence (p<0.01) of differences between actual mean RMS values in at least two levels of the "portion" effect (Figure 1). The analysis of data indicate that the mean RMS of the posterior portion of the temporalis muscle is significantly lower than RMS values of the anterior and middle portions (Table 1). In addition, means and standard deviations as well as median and median absolute deviation (MAD) values can be used as reference values for healthy people.
Means and standard deviations are more meaningful when data with normal or Gaussian distribution are analyzed, which was not observed in this data set. In view of non-normal distribution of data, we suggest the use of robust indicators such as median and MAD that can replace mean and standard deviation, respectively, in cases where outliers or deviations from normality may occur.
Graphic presentation of data distribution shows the lowest estimate of central tendency of the posterior portion of the temporalis muscle during mastication and the greatest suitability of the log-normal distribution for comparison of group means.
Discussion
In the present investigation on how the anterior, middle and posterior portions of the temporalis muscle work during mastication, it was found they all work together but with different mean electrical activity (RMS) ( Figure 1).
Although the temporalis muscle is considered unique single unit, the analysis of variance evidenced differences between mean RMS values in at least two of the three portions. Tukey's test for multiple comparisons of means showed that the posterior portion had a significantly lower activity than all other portions ( Table 1).
Several studies20-21 have described the ability of the central nervous system to activate subsets of motoneurons in the same muscle so that specific functions can be carried out. Regional differences in the histochemical composition of the fibers of the temporalis muscle have also been reported5. Neither the muscle nor its portions can function separately. By integrating the portions, depending on the motor unit firing rate and amplitude, they can have different functions.
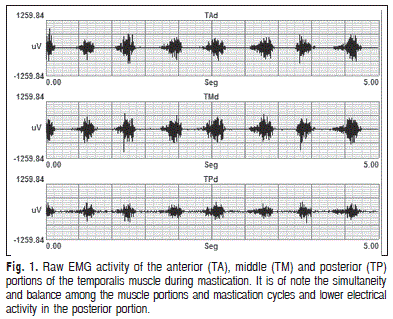
RMS values in the anterior, middle and posterior portions were 2149.77, 2531.38 and 1243.92, respectively, demonstrating that they work together with predominance of the anterior and middle portions11. However, another study found inconsistent results including similar values for the three portions4 and increased activity in the anterior and posterior portions7. These inconsistencies can probably be attributed to different methodological approaches and use of wire7 or needle4 electrodes in the tenuous and delicate fibers of the temporalis muscle, which may cause discomfort and inaccurate recording, compromising data reliability2.
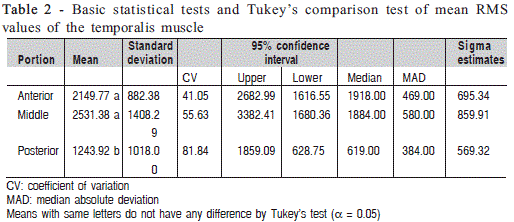
The highest RMS values were found in the anterior (2149.77) and middle (1243.92) portions of the muscle, which indicates that during mastication the temporalis muscle activity is predominantly continuous20 focused on maintaining movement. There is a predominance of type I fibers characterized by tonic contraction5 that contrast with type II fibers found in the posterior portion of the temporalis muscle that are activated during fast phasic contraction20 showing significantly lower RMS values in the present study ( Table 2).
The present results are supportive to the findings of other studies, which stated the importance of skeletal and occlusal morphological aspects of muscle activity11-13. In Angle's Class I individuals who have adequate occlusal stability and balance intermaxillary the main function of the temporalis muscle is to maintain movement. In contrast, in retrognathic individuals22, maximal voluntary contraction occurs predominantly in the posterior portion of the temporalis muscle probably to produce an antagonistic action to that of the pterygoid muscle and thereby ensure the stability of the mandible3.
In conclusion, there is an association between the portions of the temporalis muscle. It was found a significantly lower RMS value in the posterior portion showing that the anterior and middle portions of the muscle have a predominant function of maintaining movement during mastication.
References
1. Moyers R.E. An electromyographic analysis of certain muscles involved in temporomandibular movement. Am J Orthod. 1950; 36: 481-515. [ Links ]
2. Ahlgren J, Sonesson B, Blitz M. An electromyographic analysis of the temporalis function of normal occlusion. Am J Orthod. 1985; 87: 230-9.
3. Pruzansky S. The application of electromyography to dental research. J Am Dent. 1952; 44: 49-68.
4. Vitti M, Basmajian JV. Integrated actions of masticatory muscles: simultaneous EMG from eight intramuscular electrodes. Anat Rec. 1976; 187: 173-90.
5. Eriksson PO, Thornell LE. Histochemical and morphological muscle-fibre characteristics of the human masseter, the medial pterygoid and the temporal muscle. Arch Oral Biol. 1983; 28: 781-95.
6. Scott BJJ, Mason AG, Cadden SW. Voluntary and reflex control of the human temporalis muscle. J Oral Rehabil. 2002; 29: 634-43.
7. Ahlgren J, Ingervall B, Thilander B. Muscle activity in normal and postnormal occlusion. Am J Orthod. 1973; 64: 445-55.
8. Landulpho AB, Silva WABE, Silva FAE, Vitti M. Electromyographic evaluation of masseter and anterior temporalis muscles in patients with temporomandibular disorders following interocclusal appliance treatment. J Oral Rehabil. 2004; 31: 95-8.
9. Shinozaki EB, Santos MBF, Okasaki LK, Marchini L, Brugnera Junior A. Clinical assessment of the efficacy of low-level laser therapy on muscle pain in women with temporomandibular dysfunction, by surface electromyography. Braz J Oral Sci. 2010; 9: 434-8.
10. Pasinato F, Souza JA, Corrêa EC, Silva AMT. Temporomandibular disorder and generalizes joint hypermobility: electromyographic analysis of the masticatory muscles. Braz J Oral Sci. 2011; 10: 146-51.
11. Ahlgren J. EMG pattern of temporalis in normal occlusion. Europ J Orthod. 1986; 8: 185-91.
12. Subtelny JD. Malocclusions, orthodontic correction and orofacial muscle adaptation. Angle Orthod. 1970; 40: 170-201.
13. Saifuddin M, Miyamoto K, Ueda HM, Shikata N, Tanne K. An electromyographic evaluation of the bilateral symmetry and nature of masticatory muscle activity in jaw deformity patients during normal daily activities. J Oral Rehabil. 2003; 30: 578-86.
14. Hugger A, Hugger S, Schindler HJ. Surface electromyography in the assessment of the masticatory muscles for application in dental practice. Current evidence and future development. Int J Comput Dent. 2008; 11: 81-106.
15. Cram JR, Kasman GS, Holtz J. Introduction to Surface Electromyography. Gaithersburg Maryland: Aspen Publicationâ; 1998. p.257-8.
16. Fernandes MR. Estudio electromiográfico del músculo temporal. Rev Cub Estomat. 1985; 22: 177-86. 17. De Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997; 13: 135-63.
18. De Luca CJ. Electromyography. In: Webster JG, editor. Encyclopedia of Medical Devices and Instrumentation. 2nd ed. Hoboken: John Wiley and Sons; 2006. p.98-109. 19. SAS Institute Inc. The SAS System, release 9.2. SAS Institute Inc., Cary: NC.; 2008
20. Blanksma NG, Van Eijden TMGJ. Electromyographic heterogeneity in the human temporalis muscle. J Dent Res. 1990;19: 1686-90.
21. Ter Haar Romeny BM, Denier Van Der Gon JJ, Gielen CCAM. Changes in recruitment order of motor units in the human biceps muscle. Exp Neurol. 1982; 78: 360-8.
22. Lindauer SJ, Gay T, Rendell J. Effecto of jaw opening on masticatory muscle EMG force characteristics. J Dent Res. 1993; 71: 51-5.
 Correspondence:
Correspondence:
Mirian Nagae
Rua Tessália Vieira de Camargo 126
Bairro Barão Geraldo - Campinas/SP
CEP: 013.084-971 Phone: (11)83250331 (11) 32624944
E-mail: mnagae@fcm.unicamp.br
Received for publication: May 26, 2011
Accepted: September 14, 2011













