Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.10 no.3 Piracicaba Jul./Set. 2011
ORIGINAL ARTICLE
Ultrasonic irrigation in the removal of smear layer and Enterococcus faecalis from root canals
Letícia Maria Menezes NóbregaI; Cícero Romão Gadê-NetoII; Fábio Roberto DamettoII; Carlos Frederico de M. SarmentoII; Rejane Andrade de CarvalhoII
I MSc, Integrated Clinical Dentistry, Department of Dentistry, Potiguar University (Laureate International Universities), Natal, RN, Brazil
II PhD, Integrated Clinical Dentistry, Department of Dentistry, Potiguar University (Laureate International Universities), Natal, RN, Brazil
ABSTRACT
Aim: This study evaluated both smear layer removal and reduction of Enterococcus faecalis after instrumentation with ultrasonic irrigation. Methods: Root canals were experimentally inoculated with E. faecalis for 20 days and microbiological samples were collected before and after chemomechanical preparation by using sterilized absorbent paper points. The irrigation solutions used were NaOCl 2.5% and EDTA 17%. In Group 1 (G1), conventional irrigation was used, whereas in Group 2 (G2) ultrasonic irrigation was performed. In group 3 (control), root canals were irrigated with distilled water. The samples were inoculated in BHI broth and turbidity was observed after 48 h to evaluate the reduction in the number of bacteria. Residual smear layer was examined by scanning electron microscopy (SEM). Results: The results showed no significant differences between ultrasonic and conventional irrigation. Conclusions: It was concluded that the level of disinfection and cleanliness of root canals achieved with ultrasonic irrigation is comparable to that obtained by conventional methods.
Keywords: E. faecalis, endodontic irrigation, smear layer, ultrasonic.
Introduction
When endodontic infection occurs, pathogenic bacteria may spread throughout the entire root canal system1-3. The host immunological system cannot reach the bacteria present within the root canal due to an absence of local vascularization4. Elimination of infection, however, is possible via proper chemomechanical preparation (instrumentation and irrigation) and, when necessary, the use of intracanal medication5-8.
Sodium hypochlorite is one of the most commonly used agents for root canal therapy and has been proven an excellent irrigation solution due to its tissue-dissolving capacity and antibacterial action9-11.
Among the methods recommended by the current literature for smear layer removal, the use of chelating solutions, laser application and ultrasonic irrigation can be cited12-15. A number of protocols have been used in an attempt to efficiently remove smear layer, thus exposing the dentinal tubules of the root canal wall. In theory, this allows the action of intracanal medications and adequate seal by the filling material and root canal obturation. In fact, the presence of significant numbers of residual bacteria and smear layer may compromise the success of endodontic therapy16-17.
Several reports have demonstrated good results when ultrasonic irrigation is used to remove smear layer. In the mechanism being proposed, endodontic files mounted on an ultrasonic endodontic handpiece vibrate and produce streaming of the irrigation solution, which enhances elimination of debris from the root canal wall18-21. Other studies, however, have not shown statistically significant differences between conventional and sonic or ultrasonic irrigation in the removal of both smear layer and microorganisms present in the apical third of root canals22-24.
Scanning electron microscopy (SEM) analysis allows observing the cleanliness of root canal walls and has been frequently used to evaluate the cleaning efficiency of different chemomechanical preparation techniques8,11,25-28. Microbiological methods have also been used for such purpose.
The aim of this study was to evaluate in vitro the capacity of both smear layer removal and reduction of E. faecalis from root canals after instrumentation with ultrasonic irrigation.
Material and methods
Sample Preparation
Fifty-two freshly extracted human teeth with complete apex formation and similar anatomical features (single root without curvature and with uniform width and length) were divided into two groups.
All root canals were initially instrumented with manual K-files (Dentsply-Maillefer) to a size #25 master apical file and irrigated with distilled water to remove pulp tissue and to enlarge the canal for subsequent microbiological contamination. In order to eliminate the smear layer produced during this initial preparation, all roots were submitted to a bath under agitation for 10 min in 17% EDTA followed by 10 min in a 5.25% NaOCl29. The roots were then abundantly washed with distilled water to remove the EDTA and NaOCl and the apical foramens sealed with resin (Natural Flow-DFL).
The roots were immersed in glass tubes containing 5 mL of brain heart infusion broth (BHI-DIFCO) and sterilized at 121°C for 20 min. They were then kept at 37°C for 24 h to verify the success of the sterilization protocol.
Pure cultures of E. faecalis (ATCC 29212) were cultivated in BHI agar for 24 h and a suspension was prepared with turbidity corresponding to 1.0 McFarland standard (3X108 CFU/ml). The glass tubes with the sterilized roots and BHI broth were opened in a laminar flow chamber and sterile pipettes used to add 1 mL of the bacterial suspension. The tubes were kept at 37°C for 20 days, with replacement of 2.0 mL of contaminated BHI by 2.0 mL of freshly prepared BHI every 2 days to avoid medium saturation.
The turbidity of the medium during the incubation period indicated bacterial growth. The purity of the cultures was confirmed by Gram-staining and colony morphology on BHI agar.
Root Canal Preparation and Experimental Groups
All samples were shaped with ProTaper rotary files to size F5 (Dentsply-Maillefer), with the working length being determined at 1 mm from the foramen. In Group 1 (n = 19), after each instrument used, irrigation with 2 mL of 2.5% NaOCl was performed by using a disposable syringe coupled with a 27-G needle. For smear layer removal, irrigation was carried out by initial application of 1 mL of 17% EDTA for 3 min, followed by irrigation with 2 mL of 2.5% NaOCl and a final washing with 5 mL of distilled water.
In Group 2 (n = 20), after each instrument used, irrigation with 2 ml of 2.5% NaOCl was followed by passive irrigation for 15 s by using an Endo L ultrasonic handpiece (Dabi Atlante) with a size #10 K-file on a Profi II AS ceramic unit (Dabi Atlante). Passive ultrasonic irrigation is performed by applying vibrations to shake only the irrigant solution without affecting the dentin walls (15, 30). This passive irrigation was also performed after irrigation with EDTA 17%. In Group 3 (n = 13), the control group, distilled water was used for irrigation and no protocol for smear layer removal was performed.
Microbiological Samples
Microbiological samples obtained with sterile paper points were collected from contaminated root canals before and after instrumentation. After chemomechanical preparation in Groups 1 and 2, irrigation with 0.6% sodium thiosulfate solution was used to neutralize the NaOCl, followed by irrigation with 2 mL of distilled water. After collection of each sample, the paper points were transferred to tubes containing 1 mL of BHI broth, vortexed for 1 min and incubated at 37°C for 48 h. Each group had ten roots. Tubes were then examined to investigate the presence of turbidity of the medium, which was classified according to the McFarland standard scale for estimation of the number of bacteria before and after chemomechanical preparation.
Due to the great standard deviation of the turbidity value, a rank transformation was indicated for this study. It is a statistical tool that produces a table containing the ordinal rank of each value in a data set, in other words, the rank transforms the dependent variables. The Kruskal-Wallis test (Biostat 5.0 software, CNPq 2000, Brasília-DF, Brazil), a nonparametric test, was applied with a level of significance set at 5% (p < 0.05).
SEM Analysis
Immediately after root canal preparation and microbiological sampling, each root was fixed in 2.5% glutaraldehyde for 48 h. By using a diamond disc at low speed and a wedge to expose the prepared canals, the roots were then divided into two halves in a buccolingual orientation. After dehydration with increasing concentrations of alcohol, the halves selected were coated with a thin layer of gold and submitted to SEM analysis.
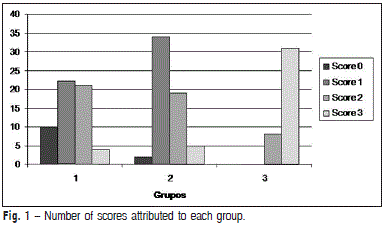
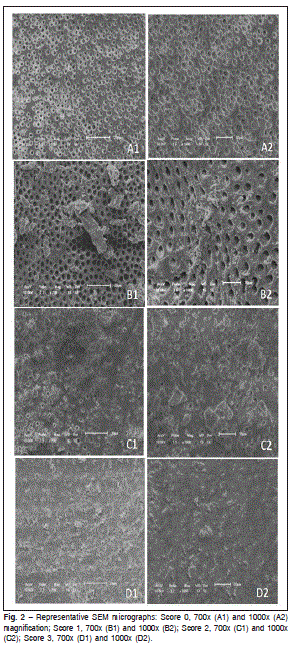
Photographs of the coronary, middle and apical thirds of the canals were taken at magnifications of 700x and 1,000x. The amount of smear layer was scored as follows: score 0 = all dentinal tubules were open and no smear layer was present; score 1 = smear layer was covering some dentinal tubules, but the majority was open; score 2 = smear layer was covering the majority of dentinal tubules; and score 3 = all dentinal tubules were covered by smear layer. These photographs were analyzed by three examiners (who had no prior knowledge on the treatment applied to each root canal) and Kappa test showed high intra and inter-examiner agreement values. Data (scores obtained) were also submitted to statistical evaluation by using the non-parametric Kruskal- Wallis test, and p values were computed and compared for statistical significance at the p < 0.05.
Results
Microbiological Samples
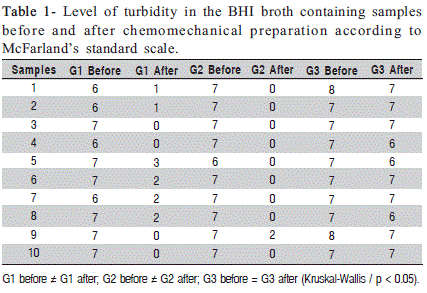
The level of turbidity of the samples before and after chemomechanical preparation is shown in Table 1. Kruskal- Wallis analysis showed a significant decrease in the turbidity, indicating reduction in the number of E. faecalis in Groups 1 and 2 (p < 0.05) regarding the samples collected before and after chemomechanical preparation. However, there was no difference between these two protocols of irrigation. Differently from Groups 1 and 2, Group 3 (control group) had only a slight decrease in the turbidity.
SEM Analysis
With respect to the smear layer removal capacity, there were no significant differences between Groups 1 and 2 (p > 0.05). The conventional and passive ultra-sonic irrigation protocols resulted in a similar satisfactory cleaning. The presence of smear layer was significantly greater in the control group (p < 0.05) and in the apical third, when compared with cervical and middle thirds. It was observed that score 3 was more frequent in Group 3 (control group), showing the least capacity to remove the smear layer. The scores for each group are presented in Figure 1. The distribution of the smear layer and their corresponding scores are depicted in representative SEM micrographs (Figure 2).
Discussion
There is a continuous search for optimal instrumentation technique and irrigation solution. A number of studies have evaluated the antimicrobial capacity of irrigation solutions and their potential advantages when used in association with other techniques. Other studies have focused on the efficacy of different instrumentation techniques and their capacity to remove debris and smear layer. The ultimate goal of such studies is to establish protocols that allow root canal walls to be as clean as possible6,8,11,13,27-31.
Indeed, ultrasonic instrumentation is not recommended because of the limited control allowed to the operator. Endodontic files do not support the propagation of vibratory energy along their central axis, which increases the risk of file breakage. Ultrasonic technology, however, may be useful for endodontic irrigation32.
A few studies have shown that ultrasonic root canal irrigation produces agitation of irrigation solutions, improving both their antimicrobial actions and smear layer removal capacity20,30. The aim of this study was to evaluate in vitro the effectiveness of passive ultrasonic irrigation during chemomechanical preparation regarding removal of smear layer and root canal decontamination.
The apical foramina of all samples were sealed to prevent extravasation of the solution and to allow it to flow optimally. Root canals were contaminated with E. faecalis not only because it is present in most endodontic infections, but also because it is routinely resistant to conventional chemomechanical preparation2-4,10,25,33.
Ultrasonic irrigation should be carried out passively without the file touching the root canal walls. Free-oscillating files are preferably used because they promote adequate agitation of the irrigation solution13-34. Thus, a K-file was adapted onto an Endo L Ultrasonic handpiece (Dabi Atlante) for the present study. Cameron12,35 evaluated ultrasonic activation for different time lengths (30 s, 1 s, 3 and 5 min). Jensen et al.14 used it for 3 min, whereas Weber et al. 30, Gutarts et al. 20 and Lui et al.21 for 1 min. It is not easy to maintain the ultrasonic file in a stationary position during an extended time period without touching the surrounding walls. In addition, this procedure can cause significant operator fatigue. Therefore, in the present study, ultrasonic irrigation was performed for 15 s after each instrumentation.
According to Abbot et al.13 and Lopes et al.7, the action of EDTA is more effective when it remains in contact with the surface for a certain period of time. EDTA should not be agitated. Thus, in Group 2, ultrasonic irrigation was carried out only after a 3 minute period of contact between EDTA and the root canal walls. In contrast, Lui et al.21 obtained better results when EDTA was a constituent of the irrigation solution used.
In the present study, ultrasonic irrigation did not clean the root canal better than conventional irrigation. Both disinfection and smear layer removal seem to be due to the use of sodium hypochlorite and EDTA as irrigant solutions, and not because of the agitation promoted by the ultrasonic irrigation13,15-17,24,36.
A number of reports have shown that the mechanical actions of irrigation alone can reduce the amount of bacteria inside the root canal5,9-10,37. In the present study, however, this was not the case. In the control group, a significant number of bacteria were still present following preparation. This may be explained by the evaluation methods used here. We carried out a qualitative analysis by assessing the bacterial growth in BHI broth and observing the medium turbidity instead of using dilution and quantification of CFUs (used in other studies).
Some studies have shown that the use of ultrasonic irrigation significantly improved disinfection and cleaning of root canal walls14,17-19,27-28,37. The results of the present study, however, did not indicate significant differences between ultrasonic and conventional irrigation. Mayer et al.22 reported similar findings.
In conclusion the results of this study suggest that the level of disinfection and cleanliness of root canals achieved with ultrasonic irrigation is comparable to that obtained by conventional methods.
References
1. Siqueira-Junior JF, Uzeda M. Influence of different vehicles on the antibacterial effects of calcium hydroxide. J Endod. 1998; 24: 653-65. [ Links ]
2. Dametto FR, Ferraz CC, Gomes BP, Zaia AA, Texeira FB, Souza-Filho FJ. In vitro assessment of the immediate and prolonged antimicrobial action of chlorhexidine gel as na endodontic irrigant against Enterococcus faecalis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005; 99: 768-72.
3. Gomes BP, Pinheiro ET, Sousa EL, Jacinto RC, Zaia AA, Ferraz CC et al. Enterococcus faecalis in dental root canal detected by culture and by polymerase chain reaction analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 102: 247-53.
4. Oliveira LD, Carvalho CAT, Jorge AOC. Microrganismos causadores de infecções pulpares e periapicais. In: Jorge AOC. Microbiologia bucal. 3. ed. São Paulo: Santos; 2007. p.127-42.
5. Siqueira-Junior JF, Lima KC, Magalhães FA, Lopes HP, Uzeda M. Mechanical reduction of the bacterial population in the root canals by three instrumentation techniques. J Endod. 1999; 25: 332-5.
6. Spratt DA, Pratten J, Wilson M, Gulabivala K. An in vitro evaluation of the antimicrobial efficacy of irrigants on biofilms of root canal isolates. Int Endod J. 2001; 34: 300-7.
7. Lopes HP, Siqueira-Junior JF, Elias CN. Substâncias químicas empregadas no preparo dos canais radiculares. In: Lopes HP, Siqueira- Junior JF, Endodontia: biologia e técnica. 2. ed. Rio de Janeiro: Medsi; 2004. p.535-79.
8. Medici MC, Froner IC. A scanning electron microscopic evaluation of different root canal irrigation regimes. Braz Oral Res. 2006; 20: 235-40.
9. Bystrom A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5% sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol. 1983; 55: 307-12.
10. Berber VB, Gomes BP, Sena NT, Vianna ME, Ferraz CC, Zaia AA, et al. Efficacy of various concentrations of NaOCl and instrumentation techniques in reducing Enterococcus faecalis within root canal and dentinal tubules. Int Endod J. 2006; 39: 10-7.
11. Vasconcelos BC, Luna-Cruz SM, De-Deus G, Moraes IG, Maniglia- Ferreira C, Gurgel-Filho ED. Cleaning ability of chlorhexidine gel and sodium hypochlorite associated or not with EDTA as root canal irrigants: a scanning electron microscope study. J Appl Oral Sci. 2007; 15: 387-91.
12. Cameron JA. The Synergistic relationship between ultrasound and sodium hypochlorite: a scanning electron microscope evaluation. J Endod. 1987; 13: 541-5.
13. Abbot PV, Heijkoop PS, Cardaci SC, Hume WR, Heithersay GS. An SEM study of the effect of different irrigation sequences and ultrasonics. Int Endod J. 1991; 24: 308-16.
14. Jensen SA, Walker TL, Hutter JW, Nicoll BK. Comparison of cleaning efficacy of passive sonic activation and passive ultrasonic activation after hand instrumentation in molar root canal. J Endod. 1999; 25: 735-8.
15. Karadag LS, Tinaz C, Mihçioglu T. Influence of passive ultrasonic activation on penetration depth of different sealers. J Contemp Dent Pract. 2004; 5: 1-7.
16. De-Deus G, Gurgel-Filho ED, Maniglia-Ferreia C, Coutinho-Filho Tauby. Influence of the filling technique on depth of tubular penetration of root canal sealer: a scanning electron microscopy study. Braz J Oral Sci. 2004; 3: 433-8.
17. Moretti AG, Pantoja CAMS, Moreira DM, Zaia AA, Almeida JFA. Effect of the smear layer on the filling of artificial lateral canals and microleakage. Braz J Oral Sci. 2011; 10: 55-9.
18. Kahn FH, Rosenberg PA, Gliksberg J. An in vitro evaluation of the irrigant characteristics of ultrasonic and subsonic handpieces and irriganting needles and probes. J Endod. 1995; 21: 277-80.
19. Lee SJ, Wu MK, Wesselink PR. The effectiveness of syringe irrigation and ultrasonics to remove debris from simulated irregularities within prepared root canal walls. Int Endod J. 2004; 37: 672-8.
20. Gutarts R, Nusstein J, Reader A, Beck M. In vivo debridement efficacy of ultrasonic irrigation following hand-rotatory instrumentation in human mandibular molar. J Endod. 2005; 31: 166-70.
21. Lui JN, Kuah HG, Chen NN. Effect of EDTA with or without surfactants or ultrasonics on removal of smear layer. J Endod. 2007; 33: 472-5.
22. Mayer BE, Peters OA, Barbakow F. Effects of rotary instruments and ultrasonic irrigation on debris and smear layer scores: a scanning electron microscopic study. Int Endod J. 2002; 35: 582–9.
23. Huffaker SK, Safavi K, Spangberg LSW, Kaufman B. Influence of a passive sonic irrigation system on the elimination of bacteria from root canal systems: a clinical study. J Endod. 2010; 36: 1315-8.
24. Pascon FM, Kantovitz KR, Puppin-Rontain RM. Influence of cleanser and irrigation om primary and permanent root dentin permeability: a literature review. Braz J Oral Sci. 2009; 5: 1063-9.
25. Yamashita JC. Avaliação, por microscopia eletrônica de varredura, da capacidade de limpeza de algumas soluções irrigadoras empregadas em endodontia [master's thesis]. Araraquara, SP: State University of São Paulo; 2000.
26. Scelza MF, Pierro V, Scelza P, Pereira M. Effect of three different time period of irrigation with EDTA-T, EDTA, and citric acid on smear layer removal. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 98: 499-503.
27. Yamashita JC, Duarte MAH, Valim FA, Almeida JM, Kuga MC, Fraga SC. Evaluation of the surface of root canal walls after utilization of endodontic rotator systems: a SEM study. J Appl Oral Sci. 2005; 13: 78-82.
28. Marque AAF, Marchesan MA, Sousa-Filho CB, Silva-Sousa YTC, Sousa- Neto MD, Cruz-Filho AM. Smear layer and chelanted calcium ion quantification of three irrigating solutions. Braz Dent J. 2006; 17: 306-9.
29. Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, Souza-Filho FJ. In vitro assessmento of the antimicrobial action and the mechanical ability of the chlorhexidine gel as an endodontic irrigant. J Endod. 2001; 27: 452-5.
30. Weber CD, McClanahan SB, Miller GA, Diener-West M, Johnson JD. The effect of passive ultrasonic activation of 2% chlorhexidine or 5,25% sodium hypochlorite irrigant on residual antimicrobial activity in root canal. J Endod. 2003; 29: 562-4.
31. Sabins RA, Johnson JD, Hellstein JW. A comparison of the cleaning efficacy of short-term sonic and ultrasonic passive irrigation after hand instrumentation in molar root canals. J Endod. 2003; 29: 674-8.
32. Nabeshima CK, Machado MLB. Avaliação da resistência de limas durante preparo ultra-sônico. Rev Assoc Paul Cir Dent. 2007; 61: 473-8.
33. Gomes BP, Souza SF, Ferraz CC, Teixeira FB, Zaia AA, Valdrighi L, Souza-Filho FJ. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecallis in bovine root dentine in vitro. Int Endod J. 2003; 35: 1-9.
34. Sluis LW, Shemesh H, Wu MK, Wesselink PR. Passive ultrasonic irrigation of root canal: a review of the literature. Int Endod J. 2007; 40: 415-26.
35. Cameron JA. The use of ultrasonic in the removal of the smear layer: a scanning electron microscope study. J Endod. 1983; 9: 289-92.
36. Chopra S, Murray P, Namerow K. A scaning electron microscope evaluation of the effectiveness of th F-files versus ultrasonic activation of K-file to remove smear layer. J Endod. 2008; 34: 1243-5.
37. Gurgel-Filho ED, Vivacqua-Gomes N, Gomes BP, Ferraz CC, Zaia AA, Souza-Filho FJ. In vitro evaluation of the effectiveness of the chemomechanical preparation against Enterococcus faecalis after singleor multiple-visit canal treatment. Braz Oral Res. 2007; 21: 308-13.
 Correspondence:
Correspondence:
Letícia Maria Menezes Nóbrega
Rua Tiradentes, 537, apto 43. Centro,
Piracicaba/SP – Brazil - 13400-760
Phone: +55 19-8223-4545 / 84-9908-7318
E-mail: letnobrega@hotmail.com
Received for publication: May 21, 2011
Accepted: September 20, 2011













