Brazilian Journal of Oral Sciences
ISSN 1677-3225
Braz. J. Oral Sci. vol.11 no.1 Piracicaba ene./mar. 2012
ORIGINAL ARTICLE
Preventive effect of an orthodontic compomer against enamel demineralization around bonded brackets
Alan Rafael Martins SavarizI; Mariana MarquezanII; Maurício MezomoIII
IGraduate Student in Orthodontics, School of Dentistry, Centro Universitário Franciscano, Brazil
IIMaster's degree in Orthodontics, Visiting professor, Graduate Program in Dentistry, School of Dentistry, Centro Universitário Franciscano, Brazil
IIIMaster's degree in Orthodontics, professor, Department of Orthodontics, School of Dentistry, Centro Universitário Franciscano, Brazil
ABSTRACT
Orthodontic appliances predispose to the accumulation of plaque due to the great number of retentive sites, which might lead to enamel demineralization adjacent to the accessories.
AIM: To assess the effectiveness of a compomer for orthodontic bonding in preventing the formation of white spots around orthodontic brackets.
METHODS: Forty extracted human premolars were divided into two groups: control group (CG), in which conventional resin Transbond™ XT Light Cure (3M Unitek™) was used to bond the brackets; and experimental group (EG), in which the compomer Transbond™ Plus Color Change (3M Unitek™) was used. pH cycling was performed for 17 days to induce the demineralization process. Enamel on the buccal face was photographed under a stereomicroscope (at 10x magnification) before (t0) and after (t1) pH cycling. The images were used to compare demineralization between the groups by using a visual scale.
RESULTS:A statistically significant difference between control and experimental groups was found (p=0.004) showing that the compomer was more efficient than the conventional resin in preventing white spots.
CONCLUSIONS: The compomer Transbond Plus Color Change was capable of inhibiting enamel demineralization adjacent to the bonding area of brackets. However, the inhibition halo did not exceed 1 mm.
Keywords: brackets, fluoride, orthodontic adhesives, tooth demineralization.
Introduction
The metabolic activity of bacteria that colonize the tooth surface causes alterations in pH, which results in an intermittent process of loss and gain of mineral of the dental tissue. Caries lesions are formed by an imbalance in the deremineralization process, when there is more frequent ion output from the dental mineral tissue, leading to the destruction of enamel1.
Orthodontic treatment with fixed appliances has been associated with an increased risk for the development of caries lesions2, since the placement of fixed appliances in the oral cavity creates new plaque-retention sites3-4, increasing significantly the number of Streptococcus mutans immediately after their placement5-6. Therefore, enamel demineralization around orthodontic accessories is a possible adverse effect of orthodontic treatment for patients with poor oral hygiene7-9.
White spots are formed after approximately 21 days of cariogenic challengeand they may be reverted by the presence of fluoride and biofilm disorganization10. Initial demineralization may be clinically detected when white opaque and rough spots appear. These spots can evolve to cavitated lesions or they can be inactivated. Inactive white spots remain as "scars" of shinny and smooth white spots11 and may compromise esthetics because they remain visible for up to 5 years after the removal of the brackets12.
Fluoride remains as the best-known cariostatic agent13. It must be constantly present in the oral cavity throughout entire orthodontic treatment to be effective in reducing demineralization around orthodontic brackets14. The use of fluoride mouthrinses with daily toothbrushing has shown positive results11,15. However, these measures have limited effectiveness since they depend on the patient's cooperation2. Therefore, the use of restorative16-18 and bonding materials19-23 that release fluoride has been an alternative to prevent caries lesions.
Fluoride has been added to the composition of resin composites, which are widely used for bracket bonding. Several commercial brands have introduced fluoridated resins and compomers on the market, but further studies are needed to prove and validate their effectiveness in preventing enamel demineralization adjacent to orthodontic accessories24.
The aim of this study was to assess in vitro the capacity of a compomer for orthodontic bonding to prevent enamel demineralization around brackets after the first days of bonding.
Material and methods
The sample was composed of 40 human premolars, previously cleaned, and stored in a glucose-free physiological solution, which were provided by the Human Tooth Bank (HTB) of the Centro Universitário Franciscano. Teeth with cracked or damaged enamel surface were excluded. The specimens were sectioned 10 mm below the cementoenamel junction with a diamond disk (H22GK-314-016™ – Komet, USA) under water cooling. The root portion was embedded in PVC tubes and filled with autopolymerizing acrylic resin.
The teeth were then randomly distributed into a control group (CG), in which Transbond™ XT Light Cure resin (3M Unitek, Monrovia, CA, USA) was used to bond the brackets, and an experimental group (EG), in which the compomer Transbond™ PlusColor Change (3M Unitek, USA) was applied (Table 1). The tooth crowns were cleaned with a rubber cup and pumice for 10 s. Then they were washed with jets of water/air and dried with compressed air for 10 s.

Bracket bonding was preceded by etching with 37% phosphoric acid gel for 15 s (3M Unitek, USA), rinsed with a air/water spray for 30 s, and dried until a characteristic frosty white etched area was observed (about 60 s). Metallic premolar brackets (Kirium™ 3M Abzil, Sumaré, SP, Brazil) were bonded on the buccal surface, parallel to the long axis of the tooth. The same amount of resin composite was used in all groups and excesses were removed with a #5 explorer with rhomboid tip. Light-polymerization was performed during for 40 s with a LED light-curing unit (Radii-cal™; SDI, Bayswater, Victoria,. Austrália) with 1,200 mw/cm2 of light intensity, maintaining a constant distance of 3 mm from material surface and angulation of 45º in relation to the tooth surface. The whole bonding procedure was performed by a single trained operator according to the manufacturers' specifications.
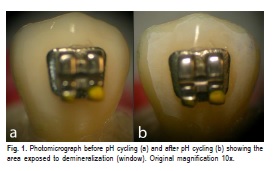
After bonding, a 2-mm window was delimited in the enamel adjacent to the brackets. The remaining buccal face was rendered waterproof with colorless nail polish (168™– Extase, Brazil). Next, the specimens were photographed under a stereomicroscope (EMF, Meiji Techno Co., Ltd, Japan) coupled to a camera (Coolpix E4500™; Nikon, Tokyo, Japan) under artificial light at 10x magnification Figure 1a). This experimental time was considered as zero (t0).
Afterwards, the samples were submitted to a pH cycling regimen at room temperature during 17 days to promote the demineralization process. First, the teeth were submerged in an artificial saliva solution [KCl 0.12%; MgCl2 0.0052%; Nipagin 0.18%; NaF 0.01%; NaCl 0.0084%; CaCl2 0.0146%; KDP 0.0342%; CMC 1%; Xilitol C 4%; NaOH 30%] at a neutral pH (7.4) for 20 h per day. They were then submerged in a demineralizing solution [KCl 0.12%; MgCl2 0052%; Nipagin 0.18%; NaF 0.01%; NaCl 0.0084%; CaCl2 0.0146%; KDP 0.0342%; CMC 1%; Xilitol C 4%; C6H8O7 50%] with pH adjusted to 4.4 for four h. After this period, the specimens returned to the neutral artificial saliva solution, giving sequence to the cycle. At change of solution, the teeth were washed with deionized water. The solutions were changed every 4 days.
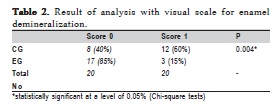
After the last cycling period, all specimens were washed with deionized water and dried with compressed air. This experimental time was considered as t1. The window working areas were photomicrographed again (Figure 1b) and the images at t0 and t1 were compared by a blinded and calibrated examiner (K=0.7). A visual scale for enamel demineralization was created for comparison between the groups, score 0 was attributed when the demineralization process was not identified in the enamel adjacent to the bonding material; and score 1 was attributed when a white spot was found adjacent to the orthodontic accessories. The data obtained were tabulated and subjected to the Chi-square test. A significance level of 5% was established.
Results
According to the visual scale for enamel demineralization, EG, in which the brackets were bonded with Transbond™ Plus Color Change, showed a larger number of specimens with score 0, which means without areas of demineralization around the brackets (p = 0.004) (Table 2).
Discussion
The results of this study showed that the compomer Transbond™ Plus Color Change was superior to the conventional resin Transbond™ XT Ligth Cure with regard to the capacity of inhibiting the formation of white spots around orthodontic brackets when the specimens were subjected to pH cycling. However, the inhibition halo was small in amplitude, not exceeding 1 mm in any of the test specimens.
Previous studies that used fluoridated materials during bracket bonding, such as fluoridated varnishes associated with conventional resins22, conventional glass ionomer cements7, resin-modified glass ionomer cements13,25 and polyacid-modified resin composites13, showed lower mineral loss around the accessories when compared with the use of conventional resins. On the other hand, other studies have shown that fluoridated adhesives do not seem to differ from conventional adhesives with regard to the capacity of preventing areas of enamel demineralization adjacent to the brackets26.
Although conventional glass ionomer cements are capable of releasing fluoride and preventing enamel demineralization adjacent to the orthodontic accessories, their bond strength is limited and they are not recommended for clinical use24,27. Thus, resin-modified glass ionomer cements and polyacid-modified resins (compomers) have been more frequently used in orthodontic clinics and research studies. When the capacity for preventing enamel demineralization around brackets of these two materials is compared, resinmodified cements have shown greater effectiveness13 because they release more fluoride13,23.
Fluoride-releasing dental materials, such as resins, cements and elastomeric ligatures, act as a reservoir of fluoride in the oral cavity and may increase the levels of fluoride in saliva, bacterial plaque and hard dental tissues. Fluoride increases enamel remineralization28 and reduces the growth of Streptococcus mutans29. The values of fluoride release from orthodontic materials vary in the literature due to methodological differences. It is still unclear which is the minimum amount of fluoride necessary to prevent demineralization around orthodontic brackets30 and the need to use fluoride in orthodontic bonding materials is controversial18.
It is known that fluoride release from dental materials occurs more markedly in the first 24 h after placement in the oral cavity, followed by an accentuated decline and tendency to stabilize over time16,25. Nevertheless, fluoridated materials present in the oral cavity can be recharged with fluoride through other means of exposure, such as toothpastes, mouthrinses and professional topical applications20. The exact mechanism of fluoride recharge is unknown17. The permeability of the material, form and concentration of fluoride are factors that might be involved in the process.
Even if fluoride-releasing orthodontic materials are used, patients should be instructed and motivated to perform oral hygiene to disorganize biofilm and use fluoridated toothpaste. Oral hygiene measures are the most effective and established methods to prevent dental caries and periodontal disease, although they depend on the patient cooperation2. Moreover, it is known that the effectiveness of fluoridereleasing orthodontic materials is limited, as demonstrated in the present study, and fluoride recharge depends on other sources of exposure, including toothpastes.
In vitro studies allow greater control of variables and are the first research tool used when a new material and its properties are tested. However, one cannot extrapolate their findings to clinical practice. Randomized controlled clinical trials are needed to confirm the relationship between compomers and lower enamel demineralization adjacent to orthodontic accessories.
In conclusion, the compomer Transbond Plus Color Change was capable of inhibiting enamel demineralization adjacent to the bonding area of brackets. However, the inhibition halo did not exceed 1 mm.
References
1. Maltz M, Carvalho J. Diagnóstico de doença cárie. In: Kriger L. ABOPREV promoção de saúde bucal. São Paulo: Artes Médicas; 1997. [ Links ]
2. Mitchell L. Decalcification during orthodontic treatment with fixed appliances—an overview. Br J Orthod. 1992; 19: 199-205.
3. Larmas M. Saliva and dental caries: diagnostic tests for normal dental practice. Int Dent J. 1992; 42: 199-208. [ Links ]
4. Pender N. Aspects of oral health in orthodontic patients. Br J Orthod. 1986; 13: 95-103. [ Links ]
5. Corbett JA, Brown LR, Keene HJ, Horton IM. Comparison of Streptococcus mutans concentrations in non-banded and banded orthodontic patients. J Dent Res. 1981; 60: 1936-42. [ Links ]
6. Mattingly JA, Sauer GJ, Yancey JM, Arnold RR. Enhancement of Streptococcus mutans colonization by direct bonded orthodontic appliances. J Dent Res. 1983; 62: 1209-11. [ Links ]
7. Geiger AM, Gorelick L, Gwinnett AJ, Griswold PG. The effect of a fluoride program on white spot formation during orthodontic treatment. Am J Orthod Dentofacial Orthop. 1988; 93: 29-37. [ Links ]
8. Lundstrom F, Krasse B. Caries incidence in orthodontic patients with high levels of Streptococcus mutans. Eur J Orthod. 1987; 9: 117-21. [ Links ]
9. Ogaard B, Rolla G, Arends J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop. 1988; 94: 68-73. [ Links ]
10. Wiltshire WA, Janse van Rensburg SD. Fluoride release from four visible light-cured orthodontic adhesive resins. Am J Orthod Dentofacial Orthop. 1995; 108: 278-83. [ Links ]
11. Benson PE, Parkin N, Millett DT, Dyer FE, Vine S, Shah A. Fluorides for the prevention of white spots on teeth during fixed brace treatment. Cochrane Database Syst Rev. 2004: CD003809. [ Links ]
12. Ogaard B. Prevalence of white spot lesions in 19-year-olds: a study on untreated and orthodontically treated persons 5 years after treatment. Am J Orthod Dentofacial Orthop. 1989; 96: 423-7. [ Links ]
13. Chin MY, Sandham A, Rumachik EN, Ruben JL, Huysmans MC. Fluoride release and cariostatic potential of orthodontic adhesives with and without daily fluoride rinsing. Am J Orthod Dentofacial Orthop. 2009; 136: 547-53. [ Links ]
14. Dijkman GE, de Vries J, Lodding A, Arends J. Long-term fluoride release of visible light-activated composites in vitro: a correlation with in situ demineralisation data. Caries Res. 1993; 27: 117-23. [ Links ]
15. Chadwick BL, Roy J, Knox J, Treasure ET. The effect of topical fluorides on decalcification in patients with fixed orthodontic appliances: a systematic review. Am J Orthod Dentofacial Orthop. 2005; 128: 601-6; quiz 70. [ Links ]
16. Aboush YE, Torabzadeh H. Fluoride release from tooth-colored restorative materials: a 12-month report. J Can Dent Assoc. 1998; 64: 561-4, 68. [ Links ]
17. Preston AJ, Higham SM, Agalamanyi EA, Mair LH. Fluoride recharge of aesthetic dental materials. J Oral Rehabil. 1999; 26: 936-40. [ Links ]
18. Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials—fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007; 23: 343-62.
19. Passalini P, Fidalgo TKS, Caldeira EM, Gleiser R, Nojima MCG, Maia LC. Preventive effect of fluoridated orthodontic resins subjected to high cariogenic challenges. Braz Dent J. 2010; 21: 211-5. [ Links ]
20. Ahn SJ, Lee SJ, Lee DY, Lim BS. Effects of different fluoride recharging protocols on fluoride ion release from various orthodontic adhesives. J Dent. 2011; 39: 196-201. [ Links ]
21. Benson PE, Shah AA, Millett DT, Dyer F, Parkin N, Vine RS. Fluorides, orthodontics and demineralization: a systematic review. J Orthod. 2005; 32: 102-14. [ Links ]
22. Behnan SM, Arruda AO, Gonzalez-Cabezas C, Sohn W, Peters MC. In-vitro evaluation of various treatments to prevent demineralization next to orthodontic brackets. Am J Orthod Dentofacial Orthop. 2010; 138: 712 e1-7; discussion 712-3. [ Links ]
23. Rix D, Foley TF, Banting D, Mamandras A. A comparison of fluoride release by resin-modified GIC and polyacid-modified composite resin. Am J Orthod Dentofacial Orthop. 2001; 120: 398-405. [ Links ]
24. Rogers S, Chadwick B, Treasure E. Fluoride-containing orthodontic adhesives and decalcification in patients with fixed appliances: a systematic review. Am J Orthod Dentofacial Orthop. 2010; 138: 390 e1-8; discussion 90-1. [ Links ]
25. Paschos E, Kleinschrodt T, Clementino-Luedemann T, Huth KC, Hickel R, Kunzelmann KH, et al. Effect of different bonding agents on prevention of enamel demineralization around orthodontic brackets. Am J Orthod Dentofacial Orthop. 2009; 135: 603-12. [ Links ]
26. Leizer C, Weinstein M, Borislow AJ, Braitman LE. Efficacy of a filledresin sealant in preventing decalcification during orthodontic treatment. Am J Orthod Dentofacial Orthop. 2010; 137: 796-800. [ Links ]
27. Millett DT, McCabe JF. Orthodontic bonding with glass ionomer cement—a review. Eur J Orthod. 1996; 18: 385-99.
28. Forss H, Seppa L. Prevention of enamel demineralization adjacent to glass ionomer filling materials. Scand J Dent Res. 1990; 98: 173-8. [ Links ]
29. Friedl KH, Schmalz G, Hiller KA, Shams M. Resin-modified glass ionomer cements: fluoride release and influence on Streptococcus mutans growth. Eur J Oral Sci. 1997; 105: 81-5. [ Links ]
30. Hellwig E, Lussi A. What is the optimum fluoride concentration needed for the remineralization process? Caries Res. 2001; 35 Suppl 1:57-9. [ Links ]
 Correspondence:
Correspondence:
Maurício Mezomo
Rua Alberto Pasqualini – 70 / 809
Zip code: 97015-010
Santa Maria-RS, Brazil
E-mail: mezomo@ortodontista.com.br
Received for publication: July 04, 2011
Accepted: January 03, 2012