Brazilian Journal of Oral Sciences
ISSN 1677-3225
Braz. J. Oral Sci. vol.11 no.1 Piracicaba ene./mar. 2012
ORIGINAL ARTICLE
Clinical effects of supragingival plaque control on uncontrolled type 2 diabetes mellitus subjects with chronic periodontitis
Andrea SonI; Claudia PeraII; Paulo UedaII; Renato Corrêa Viana CasarinIII; Suzana Peres PimentelIII; Fabiano Ribeiro CiranoIII
IDDS student, Paulista University, Brazil
IIDDS, MSc student, Periodontics Division, Paulista University, Brazil
IIIDDS, MSc, PhD, Professor, Periodontics Division, Paulista University, Brazil
ABSTRACT
AIM: To determine the clinical changes occurred in chronic periodontitis patients presenting uncontrolled type 2 diabetes mellitus after a supragingival plaque control period.
METHODS: Subjects presenting generalized chronic periodontitis were divided into two groups: Non-diabetics (n=20) – healthy subjects presenting chronic periodontitis; and Diabetics (n=14) – subjects with uncontrolled type 2 diabetes mellitus presenting chronic periodontitis. All subjects went through 28 days of supragingival plaque control - ST - (including prophylaxis, calculus removal, extraction of hopeless teeth and oral hygiene instructions) and were evaluated at baseline and after 28 days by the following parameters: Full-Mouth Plaque Score (FMPS) and Full-Mouth Bleeding Scores (FMBS), Periodontal Probing Depth (PPD), Gingival Recession (GR) and Clinical Attachment Level (CAL). ANOVA/Tukey's test and Student's t test were used for data analysis.
RESULTS: No statistically significant differences (p>0.05) between groups were observed at baseline for any parameter. Both groups presented a significant reduction in FMPS and FMBS after 28 days (p<0.05), but no statistically significant difference was found (p>0.05) between groups. Clinically, only the Non-diabetic group showed a significant improvement after ST, in PPD of initially deep pockets (p<0.05). However, no change in the clinical parameters was observed in the diabetic subjects (p>0.05).
CONCLUSIONS: It may be concluded that uncontrolled diabetes mellitus reduces periodontal changes in the supragingival plaque control regimen of subjects presenting with chronic periodontitis.
Keywords: diabetes mellitus, plaque control, chronic periodontitis.
Introduction
Chronic periodontitis results from the presence of complex microbial communities in the subgingival sulcus1, and diabetes mellitus, especially if poorly controlled, increases significantly risk for development of extensive and severe diseases2. Hyperglycemia and resultant advanced glycation end product formation, which is one of several pathways thought to lead to the vascular complications with diabetes, are also involved in the pathophysiology of periodontitis in diabetic subjects3, leading to an imbalanced release of pro- and anti-inflammatory cytokines4-6 and osteoclastogenesis-related factors7.
Despite the differences in pathogeneses, biofilm still remains the primary etiologic factor for the development of a destructive periodontal disease2. Thus, the primary goal of periodontal therapy is to target the subgingival biofilm present in periodontally diseased sites that are associated with the progressive destruction of the supportive periodontal tissues. It is well documented that conventional therapy, i.e., subgingival scaling and root planning, is effective in achieving this goal. However, supragingival plaque control appears to have a significant effect on clinical and microbiological characteristics of periodontal pockets, which could be associated with the close relationship between those environments8.
Previous studies have evaluated the relationship between supragingival plaque control and clinical and microbiological effects on subgingival areas, reporting a positive effect in systemically healthy subjects with periodontitis, i.e., a reduction in probing depth and some periodontal pathogens and preventing re-colonization9-11. However, conflicting results of the impact of supragingival dental biofilm control on clinical features in untreated periodontal sites are found in the literature12-14.
In this context, there is an interest in the possible effect of supragingival biofilm control on the subgingival environment in untreated periodontitis sites in diabetic patients, since, in previous studies, these patients presented with some altered biofilm compositions, with a higher prevalence of periodontal pathogens. Thus, the aim of the present study was to determine clinical changes in type 2 diabetic patients after 28 days of strict supragingival plaque control compared with non-diabetic patients.
Material and methods
Population Screening
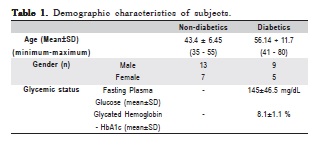
Initially, manuscript design was approved by the institutional Ethics Committee (protocol number 014/09). Eligible patients were selected from those referred to the Graduate Clinic of Paulista University, Brazil. All patients received a complete periodontal examination, including full mouth periodontal probing, radiographic examination, and complete clinical interview. Moreover, type 2 diabetic patients were sent to the clinic by Vila Mariana Health Center (HCVM), São Paulo, Brazil after being diagnosed using the Fasting Plasma Glucose (FPG) > 110 mg/dL and the glycated
hemoglobin (Hba1c) > 7% in two different examinations. All diabetic subjects were followed by a physician at HCVM. Subjects who did not have diabetes but who presented with periodontitis were also selected in order to compare of the clinical response of both types of patients.
The study inclusion criteria were the following: Diagnosis of chronic periodontitis, according to the criteria of the 1999 international classification15; at least 8 teeth with a periodontal probing depth (PPD) > 5 mm and bleeding on probing; presence of at least 20 teeth; and good general health. Patients who were pregnant or lactating, required antimicrobial premedication for the performance of periodontal examination and treatment, received a course of periodontal treatment within the last 6 months, smokers, those under use of longterm antiinflammatory drugs, suffered from any other systemic diseases (cardiovascular, pulmonary, liver, and cerebral diseases), or had received antimicrobial treatment in the previous 3 months were excluded from the study.
The sample size was determined after considering data in the literature and was aimed at obtaining a minimum power value of 0.8 to detect a difference and 0.8 mm between groups in clinical attachment level (CAL) (primary variable). A blinded and calibrated examiner was used (intra-class correlation for CAL) = 94% in a parallel design.
Supragingival Plaque Control therapy (ST)
After full mouth examination and participants' informed consent, the patients in both groups received a full mouth prophylaxis, supragingival calculus, and biofilm removal using Gracey curettes, ultrasonic scaler, bicarbonate spray, and dental floss. Also, condemned teeth were extracted and biofilm retentive factors were removed. Moreover, the patients were individually instructed on how to perform oral selfcare, including the Bass technique, inter-dental flossing, and tongue brushing. All subjects received a standard fluoride dentifrice, toothbrushes, and dental floss as necessary and were asked to perform complete oral self-care hygiene at least twice a day. A week after this first instruction session, patients returned for reinforcement of the oral self-care instructions. Twenty-eight days after ST, clinical re-evaluation was performed.
Groups
Subjects were distributed to the following groups: Diabetics (N=14): composed of individuals presenting with uncontrolled type 2 diabetes mellitus and generalized chronic periodontitis and Non-Diabetics (N=20): composed of individuals presenting with generalized chronic periodontitis.
Clinical Parameters
The following clinical parameters were assessed immediately before and 28 days after plaque control therapy using a PCP-15 periodontal probe (Hu-friedy, Chicago, IL, USA): Full-mouth Plaque Index (FMPI)16 and Full-Mouth Bleeding Score (FMBS)17; represented by the percentage of positive sites; PPD – Distance from the bottom of the pocket to the gingival margin); Gingival Recession (GR – distance from the gingival margin to cement-enamel junction); CAL – distance from the bottom of the pocket to cement-enamel junction).
Glycemic status
A single laboratory performed the glycated hemoglobin (HbA1c) and fasting plasma glucose (FPG) tests in order to confirm Diabetes mellitus status. HbA1c (%) was measured using high-performance liquid chromatography, and FPG was performed using the glucose oxidase method. Foruncontrolled cut-off, HbA1c should be higher than 7% and FPG > 120 mg/dL. All patients were under physician monitoring and taken oral hypoglycemic pills.
Data Management and Statistical Analysis
TFor clinical parameters, a repeated-measures analysis of variance (ANOVA) was used to detect intra-group differences in clinical parameters (GR, PPD, CAL), considering the patient as a statistical unit. When a statistical difference was found, the analysis of the difference was determined using the Tukey's test. The Friedman test was used to detect intragroup differences and Kruskal-Wallis was used for the intergroup analysis of the Full Mouth Plaque and Bleeding Index among all periods. The level of significance was set at 5%.
Results
Table 1 displays the demographic, clinical, and diabetic status of the population included in the present study. No difference was found regarding gender, age, and clinical parameters (PPD, CAL). Moreover, the FPG and HbA1c levels of diabetic subjects evidenced their poor glycemic control.
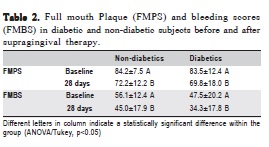
Table 2 shows changes in plaque and bleeding indices after supragingival plaque control. Both groups exhibited a reduction in plaque and bleeding (p<0.05) and no significant difference between them was observed at baseline or after the supragingival plaque control period (p>0.05).
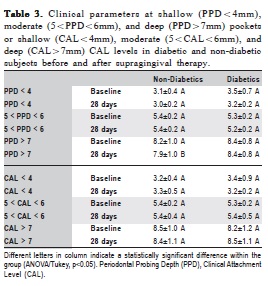
Table 3 shows the clinical changes of shallow (PPD<4mm), moderate (PPD = 5-6 mm), and deep pockets (PPD>7mm) after 28 days of strict plaque control.
At shallow and moderate pockets, no statistically significant change could be seen regarding any clinical parameter (p>0.05). However, in relation to deep pockets, a significant reduction in PPD was observed in non-diabetic individuals, although no statistical changes were observed regarding CAL. At the same time, no significant change in PPD and CAL was detected in the diabetic participants, indicating that supragingival plaque control did not promote significant changes in these subjects.
Discussion
Periodontal disease and diabetes mellitus belong to a pathologic condition in which both diseases could negatively interfere with each other, constituting a bidirectional relationship2. Diabetes mellitus, especially when uncontrolled, appears to be an important risk factor for periodontal destruction, since an alteration in host-response and microbiological aspects occurs in diabetic subjects. Periodontal disease led to an increase in insulin resistance, which could impair glycemic control. This way, the control of periodontal disease is necessary for better systemic health in these individuals. Periodontal treatment relies on biofilm disruption and plaque control to prevent recolonization and recurrence of the disease. For this, an essential phase of this treatment is supragingival plaque control. So, the present study evaluated the periodontal changes in type 2 diabetic subjects and non-diabetic subjects after a supragingival plaque control regimen. Diabetics did not show significant changes in their clinical aspects, although in non-diabetic subjects, a statistically significant change could be seen, especially in deep pockets.
Diabetic subjects with poor glycemic control present an altered release of pro and anti-inflammatory cytokines4. Moreover, some previous studies have shown that the microbiota associated with diabetes does not appear different from the microbiota of non-diabetic patients18-22. Glucose concentration in gingival crevicular fluid has been correlated with a high glucose concentration in the serum23. Elevated glucose levels in saliva and gingival crevicular fluid could induce an increase in the number of saccharolytic bacteria associated with dental caries in the saliva and in the supragingival and subgingival plaque of diabetic patients24, which could modify the biodiversity in diabetic subjects. Moreover, glycemic control could contribute to this harboring-microbiota profile, since different levels of plasma glucose may also indicate different levels of this carbohydrate in the subgingival area, probably altering environmental and microbiota characteristics. These patterns together make uncontrolled diabetes mellitus an important modifier of periodontal tissues and could impair periodontal response to treatment and be associated with this absence of significant changes after a supragingival plaque control regimen. At the same time, clinical improvement after supragingival plaque control was observed in non-diabetic subjects. Corroborating with our study, previous clinical and microbiological trials showed that supragingival plaque control are able to promote significant changes in periodontal conditions. Ribeiro et al.11 observed a gingival inflammation reduction, corroborating other studies that show a positive influence in shallow and moderate pockets9-10, although some studies also showed a benefit in deep pockets25-26. In sequential studies, Gomes et al.26-27 identified some clinical and microbiological improvement when supragingival plaque control was performed for 6 months. Although no statistically significant reduction has been observed regarding specific periodontal pathogens (Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythia ), the total number of subgingival bacteria were reduced when supragingival was performed27.
More recently, Melmann et al.28, evaluate the impact of supragingival plaque control, following the same protocol used in the present study, a microbiological positive effect was observed, using a 16S cloning technique for biodiversity analysis. In this study, a reduction in some phylotypes was observed in higher frequency in non-smokers than smokers, especially those genera known as periodontal pathogens or associated to sites presenting periodontal destruction28. It could indicate that some systemic modifiers, such as smoking in Melmann's study and type 2 diabetes mellitus in our study, could depreciate the periodontal and microbiological changes occurring after supragingival plaque control, once in both studies normal subjects presented better results. It could be seen when considering subgingival periodontal treatment, when both smokers and type 2 diabetic subjects have a worse periodontal response than healthy individuals29-30. Although Gomes et al.26-27 showed similar response of smokers, it is important to have in mind that those authors used a 3-month period of supragingival plaque control with reinforcement in oral hygiene instruction, while in Melmann's study28 and in the present study a 28-day supragingival plaque control regimen was applied. It could represent a possible effect of supragingival plaque control protocol on clinical and microbiological results, what should be addressed in future trials. In the present study, the removal of plaque retentive factors and oral hygiene instructions was carried out in one session. Plaque control during the next 28 days was the responsibility of the patient, with reinforcement in oral hygiene methods at 15 days. The protocol of one session of supragingival plaque control was chosen because of the difficulty in making patients return 3 times per week for professional plaque control. This way, further evaluation in diabetic subjects employing different plaque control regimens should be done.
Considering the results of the present study, it may be concluded that uncontrolled diabetes mellitus reduces periodontal changes in the supragingival plaque control regimen of subjects presenting with chronic periodontitis.
Acknowledgements
This study was supported by National Council for Scientific and Technological Development (PIBIC/CNPq - Grant 154857/2010-6).
References
1. Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005; 38: 135-87. [ Links ]
2. Mealey BL. Periodontal disease and diabetes. A two-way street. J Am Dent Assoc. 2006; 137 (Suppl): 26S-31S [ Links ]
3. Mealey BL, Rose LF. Diabetes mellitus and inflammatory periodontal diseases. Curr Opin Endocrinol Diabetes Obes. 2008; 15: 135-41. [ Links ]
4. Vieira Ribeiro F, de Mendonça AC, Santos VR, Bastos MF, Figueiredo LC, Duarte PM. Cytokines and bone-related factors in systemically healthy patients with chronic periodontitis and patients with type 2 diabetes and chronic periodontitis. J Periodontol. 2011; 82: 1187-96. [ Links ]
5. Santos VR, Ribeiro FV, Lima JA, Napimoga MH, Bastos MF, Duarte PM. Cytokine levels in sites of chronic periodontitis of poorly controlled and well-controlled type 2 diabetic subjects. J Clin Periodontol. 2010; 37: 1049-58. [ Links ]
6. Shin DS, Park JW, Suh JY, Lee JM. The expressions of inflammatory factors and tissue inhibitor of matrix metalloproteinase-2 in human chronic periodontitis with type 2 diabetes mellitus. J Periodontal Implant Sci. 2010; 40: 33-8. [ Links ]
7. Duarte PM, de Oliveira MC, Tambeli CH, Parada CA, Casati MZ, Nociti FH-Jr. Overexpression of interleukin-1beta and interleukin-6 may play an important role in periodontal breakdown in type 2 diabetic patients. J Periodontal Res. 2007; 42: 377-81. [ Links ]
8. Listgarten MA, Mayo HE, Tremblay R. Development of dental plaque on epoxy resin crowns in man. A light and electron microscopic study. J Periodontol. 1975; 46: 10-26. [ Links ]
9. Hellström MK, Ramberg P, Krok L, Lindhe J. The effect of supragingival plaque control on the subgingival microflora in human periodontitis. J Clin Periodontol. 1996; 23: 934-40. [ Links ]
10. Westfelt E, Rylander H, Dahlén G, Lindhe J. The effect of supragingival plaque control on the progression of advanced periodontal disease. J Clin Periodontol. 1998; 25: 536-41. [ Links ]
11. Ribeiro Edel P, Bittencourt S, Nociti-Júnior FH, Sallum EA, Sallum AW, Casati MZ. The effect of one session of supragingival plaque control on clinical and biochemical parameters of chronic periodontitis. J Appl Oral Sci. 2005; 13: 275-9. [ Links ]
12. Kho P, Smales FC, Hardie JM. The effect of supragingival plaque control on the subgingivalmicroflora. J Clin Periodontol. 1985; 12: 676-86. [ Links ]
13. Beltrami M, Bickel M, Baehni PC. The effect of supragingival plaque control on the composition of the subgingival microflora in human periodontitis. J Clin Periodontol. 1987; 14: 161-4. [ Links ]
14. McNabb H, Mombelli A, Lang NP. Supragingival cleaning 3 times a week. The microbiological effects in moderately deep pockets. J Clin Periodontol. 1992; 19: 348-56. [ Links ]
15. Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999; 4: 1-6. [ Links ]
16. Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975; 25: 229-35. [ Links ]
17. Mühlemann HR, Son S. Gingival sulcus bleeding—a leading symptom in initial gingivitis. Helv Odontol Acta. 1971; 15: 107-13
18. Katz PP, Wirthlin MR Jr, Szpunar SM, Selby JV, Sepe SJ, Showstack JA. Epidemiology and prevention of periodontal disease in individuals with diabetes. Diabetes Care. 1991; 14: 375-85. [ Links ]
19. Yuan K, Chang CJ, Hsu PC, Sun HS, Tseng CC, Wang JR. Detection of putative periodontal pathogens in non-insulin-dependent diabetes mellitus and non-diabetes mellitus by polymerase chain reaction. J Periodontal Res. 2001; 36: 18-24. [ Links ]
20. Tervonen T, Oliver RC, Wol LF, Bereuter J, Anderson LA, Aeppli DM. Prevalence of periodontal pathogens with varying metabolic control of diabetes mellitus. J Clin Periodontol. 1994; 21: 375-9. [ Links ]
21. Sbordone L, Ramaglia L, Barone A, Ciaglia RN, Tenore A, Iacono VJ. Periodontal status and selected cultivable anaerobic microflora of insulindependent juvenile diabetics. J Periodontol. 1995; 66: 452-61. [ Links ]
22. Zambon JJ, Reynolds H, Fisher JG, Shlossman M, Dunford R, Genco RJ. Microbiological and immunological studies of adult periodontitis in patients with noninsulin-dependent diabetes mellitus. J Periodontol. 1988; 59: 23-31. [ Links ]
23. Ficara AJ, Levin MP, Grower MF, Kramer GD. A comparison of the glucose and protein content of gingival fluid from diabetics and non diabetics. J Periodontal Res. 1975; 10: 171-5. [ Links ]
24. Hintao J, Teanpaisan R, Chongsuvivatwong V, Ratarasan C, Dahlen G. The microbiological profiles of saliva, supragingival and subgingival plaque and dental caries in adults with and without type 2 diabetes mellitus. Oral Microbiol Immunol. 2007; 22: 175-81. [ Links ]
25. Nogueira Moreira A, Luna Davila G, Bianchini H, Alonso C, Piovano S. Effect of supragingival plaque control on subgingival microflora and gingivoperiodontal tissues. Acta Odontol Latinoam. 2000; 13: 73-86. [ Links ]
26. Gomes SC, Piccinin FB, Susin C, Oppermann RV, Marcantonio RA. Effect of supragingival plaque control in smokers and never-smokers: 6-month evaluation of patients with periodontitis. J Periodontol. 2007; 78: 1515-21. [ Links ]
27. Gomes SC, Nonnenmacher C, Susin C, Oppermann RV, Mutters R, Marcantonio RA. The effect of a supragingival plaque-control regimen on the subgingival microbiota in smokers and never-mokers: evaluation by real-time polymerase chain reaction. J Periodontol. 2008; 79: 2297-304. [ Links ]
28. Meulman TB, Casarin RC, Peruzzo DC, Giorgetti AP, Barbagallo A, Casati MZ, et al. Impact of supragingival therapy on subgingival microbial profile in smokers versus non-smokers with severe chronic periodontitis. J Oral Microbiol. 2012; 4: doi: 10.3402/jom.v4i0.e8640. [ Links ]
29. Nassarawin NA. Effect of smoking on the response to nonsurgical periodontal therapy. East Mediterr Health J. 2010; 16: 162-5. [ Links ]
30. Kudva P, Tabasum ST, Garg N. Evaluation of clinical and metabolic changes after non-surgical periodontal treatment of type 2 diabetes mellitus patients: a clinico biochemical study. J Indian Soc Periodontol. 2010; 14: 257-62. [ Links ]
 Correspondence:
Correspondence:
Fabiano Ribeiro Cirano
Periodontics Division, Paulista University
Av Dr Bacelar,1212, Vila Clementino, São Paulo,
São Paulo - CEP 04026-002
E-mail: cirano@unip.com.br
Received for publication: November 11, 2011
Accepted: February 08, 2011