Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.11 no.2 Piracicaba Abr./Jun. 2012
ORIGINAL ARTICLE
Cytotoxic response of two cell lines exposed in vitro to four endodontic sealers
Camilla Christian Gomes MouraI; Natássia Cristina Martins OliveiraII; Cláudia Renata Bibiano BorgesIII; Maria Aparecida de SouzaIV; João Carlos Gabrielli BiffiV
IDDS, MSc, PhD, Postdoctoral Student, School of Dentistry, Federal University of Uberlândia, Uberlândia, MG, Brazil
IIDDS, MSc, PhD student, Piracicaba Dental School, UNICAMP – University of Campinas, Piracicaba, SP, Brazil
IIIMSc, PhD student in Pathology, Department of General Pathology, Federal University of Triângulo Mineiro, Uberaba, MG, Brazil
IVMSc, PhD, Researcher, Department of Applied Immunology and Parasitology, Federal University of Uberlândia, Uberlândia, MG, Brazil
VDDS, MSc, PhD, Professor, Department of Endodontics, School of Dentistry, Federal University of Uberlândia, Uberlândia, MG, Brazil
ABSTRACT
AIM: To investigate the cytotoxicity of four endodontic sealers with different bases – Epiphany (EPH), AH Plus (AHP), Sealer 26 (S26) and Endofill (ENF) – on human foreskin fibroblasts (HFF) and mouse macrophages (J774/G8).
METHODS: Cells were placed in direct contact with freshly prepared endodontic sealers in polypropylene tubes. The cells were incubated for 24, 48 and 72 h. Cytotoxicity was assessed using the MTT assay (cell viability) and Griess reagent (NO release).
RESULTS: On the HFF cultures, EPH showed the lowest viability levels of all four sealers at 24 h (p<0.05), but over time (72h), EPH lessened its toxic levels in a similar pattern as the other three materials (p>0.05). The viability of all four sealers on the macrophage cultures showed no statistically significant difference over time, except between EPH and AHP at 72 h (p<0.05). Although uniformity was not detected in macrophage and fibroblast release of NO in response to sealers over time, a trend of increased NO levels for EPH (p<0.05) was observed.
CONCLUSIONS: The response pattern varied depending on time and type of cell line used for analysis, although the results indicate a higher cytotoxicity for EPH in short-term tests.
Keywords: cell culture, cytotoxicity, fibroblast, macrophage, root canal sealers.
Introduction
A root canal sealer should be chosen based on its biological1 and physicochemical characteristics, ability to adhere to and seal the root canal system, dimensional stability, nonabsorbability, radiopacity, and adequate working time2-3. Although root-filling materials are designed for use only within the canal space, leakage through the apical constriction may occur, allowing the periradicular tissues to come in contact with the toxic components of the sealer4. Furthermore, the induction of cell death caused by these materials, which is associated with the release of proinflammatory mediators, leads to a persistence of periapical inflammatory reaction and increases the time required for wound healing5. Hence, it is important to comprehensively evaluate the biocompatibility of different sealers.
This type of analysis can be conducted both in vitro and in vivo. However, in vitro studies are faster and less expensive than in vivo tests; furthermore, factors and variables may be controlled in vitro6. In spite of the advantages of the in vitro tests, they are not able to mimic the orchestrated role of cells present inperiradicular region and the long-term cytotoxicity presented by the sealers. Cytotoxicity assays are the initial screening tests used to evaluate the biocompatibility of materials7. Genotoxicity/mutagenicity/carcinogenicity and microbial effects are the other parameters that characterize biocompatibility8. The cytotoxic responses to different root canal sealers vary considerably, depending on the sealer's chemical composition4, namely zinc oxide eugenol, calcium hydroxide, mineral trioxide aggregate9, glass-ionomer or polymers (i.e., epoxy resins, polydimethylsiloxane and methacrylates).
Although the cytotoxicity of endodontic sealers has been extensively investigated, most previous studies have used fibroblast cell lines.4,6,8,10-11 The behavior of other inflammatory cells present in the periapical region has not been widely assessed, although they also contribute to the intensity of the biological response to these materials. In order to take a new approach to previous in vitro studies on the cytotoxicity of endodontic materials, it would be interesting to evaluate the response of fibroblasts and macrophages, which are cell lines involved in periapical inflammation and repair.
Macrophages are the prevalent cells in inflammatory infiltrates that respond to sealers12-13. They also play a key role in defense and repair by producing a myriad of substances with inflammatory activity12-14. Nitric oxide (NO) is a pro-inflammatory compound that plays an important role in the investigation of a sealer's cytotoxicity13,15 because it is produced by various cells others than macrophages, such as fibroblasts16. Given that these two cell lines are widely distributed on periradicular tissues and that their response can be influenced by the stage-setting of the sealing material, the aim of this study was to investigate the cytotoxicity of four endodontic sealers – a multi-methacrylate resin-based (Epiphany), an epoxy resin-based (AH Plus), an epoxy resin and calcium hydroxide-based (Sealer 26) and a zinc oxide eugenol-based (Endofill) – with respect to the cell viability and NO release in immortalized cell cultures of macrophages and fibroblasts.
Material and methods
Root canal sealers and Sample Preparation
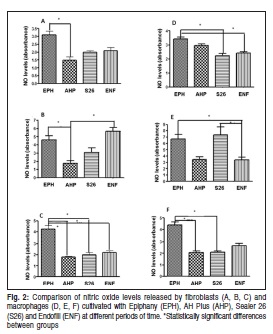
Four classes of endodontic sealers were evaluated in this study ( Table 1). Sealers were prepared according to the manufacturers' instructions, under aseptic conditions, prior to insertion into a sterile 1-mm-diameter urethral polypropylene tube (Medsonda, Arapoti, PR, Brazil). The tube was cut into 10-mm-long segments. The experiments were performed at two different days on four tubes per day for a total of eight samples per group in each experimental time. Sealers were prepared immediately before they were introduced into cell cultures in order to simulate fresh conditions.
Cell Cultures
Two cell lines were used to evaluate the endodontic sealers: human foreskin fibroblasts (HFF, Cell Bank of Rio de Janeiro, Rio de Janeiro, RJ, Brazil) and mouse macrophages (J774/G8, System Biosciences, Mountain View, CA, USA). The two cell lines were cultivated separately. The cells were routinely maintained in Dulbecco's modified Eagle's medium (DMEM) (Vitrocell, Campinas, SP, Brazil), supplemented with a 5% fetal calf serum (Laborclin, Pinhais, PR, Brazil), 100 U/mL penicillin, 100 μg/mL streptomycin and 2 mM Lglutamine (Cambrex Bio Science, Verviers, Belgium) at 37ºC in a humidified atmosphere of 5% CO2 and 95% air. The cells were plated at 2x104 cells/well in a 48-well plate that was constantly in contact with the culture medium in which the polypropylene tubes containing the endodontic sealers were placed. After incubation for 24, 48, and 72 h, cell viability assays and NO quantification within the supernatant were performed on each cell line at each experimental time. Culture wells containing empty tubes were filled with the culture medium. The median absorbance of these wells was subtracted from the samples in cell viability assays.
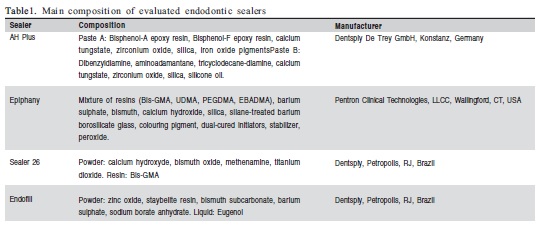
Cell Viability
Cell viability was evaluated using a MTT [3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Succinate dehydrogenase activity was determined by adding 40 μL of a 5 mg/mL MTT salt (M-2128, Sigma- Aldrich, St Louis, MO, USA) to each well and incubating the cells at 37°C for 4 h. After incubation, the resulting formazan crystals were dissolved by adding 400 μL of DMSO (Labsynth, Diadema, SP, Brazil). Then, a 100 ìL aliquot of this solution was transferred to separate wells of a 96-well ELISA plate (Corning Costar, Corning, NY, USA), and the absorbance was measured at 570 nm using a microplate reader (Instrutherm spectrophotometer UV-2000A, São Paulo, SP, Brazil). The optic density (OD) of each well was proportional to the amount of coloring.
Determination of NO levels
The production of NO was determined by measuring the accumulation of nitrite (NO2 ¯), a stable metabolite of NO, in culture supernatants by using a colorimetric reaction with Griess reagent (0.1% N-(1-naphthyl) ethylenediamine dihydrochloride, 1% sulfanilamide and 2.5% H3PO4). In each well, an equal volume of culture supernatant and Griess reagent were mixed and incubated in the dark for 10 min at room temperature. Absorbance was measured with a microplate reader at 570 nm (Instrutherm spectrophotometer UV-2000A). The concentration of nitrite in the samples was determined from a sodium nitrite (NaNO2) standard curve (200 μmol).
Statistical analysis
The statistical analysis was performed with the GraphPad Software (GraphPad Inc., San Diego, CA, USA). The Kruskal-Wallis test and post hoc Dunn's multiple-comparison test were performed to compare data from cell viability. The oneway analysis of variance and Tukey's multiple-comparison test were used to compare data from NO release. Differences were considered significant at p<0.05.
Results
Cell viability
The MTT analysis of cell viability within 24 h showed a statistically significant difference between the HFF fibroblasts that were cultivated in contact with EPH and the fibroblasts that were in contact with other sealers (AHP p<0.01; S26 p<0.001; ENF p<0.001, Figure 1A). At 48 h, only the fibroblasts that were cultured in contact with EPH and AHP showed statistically significant differences for viability (p<0.05, Figure 1B). At 72 h, there were no significant differences between the fibroblasts that were cultivated in contact with any of the four root canal sealers (p>0.05, Figure 1C). The J774/G8 macrophage showed no significant differences in viability when cultured with any of the four sealers at 24 h (p=0.1202, Figure 1D) or 48 h (p=0.6098, Figure 1E). In these cells, the absorbance levels regarding cell viability only showed significant differences at 72h in comparison to the cells that were cultivated in contact with EPH and AHP (p=0.020, Figure 1F).
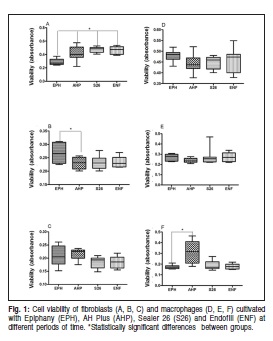
Determination of NO levels
The nitrite levels that were produced by the HFF cells in contact with the four sealers showed EPH to be the most cytotoxic. At 24 h, EPH caused a greater release of nitrite by HFF, which was significant when compared with the cells that were cultivated with AHP (p<0.001, Figure 2A). However, no significant difference was found between the other two sealers in the same period (p>0.05). At 48 h, there were significant differences in the nitrite levels produced by the fibroblasts that were cultured in contact with EPH and AHP (p<0.05) and AHP and ENF (p<0.01), while the ENF produced more of this metabolite ( Figure 2B). At 72 h, the cells cultured with EPH released nitrite levels that were significantly higher than the levels released by the cells cultured with the other sealers (AHP p<0.001; S26 p<0.001; ENF p<0.05, Figure 2C).
The levels of nitrite that were released by macrophages showed a different pattern of HFF with significant differences for the different sealers at each experimental time (p<0.05). At 24 h, EPH showed higher average levels of nitrite than the other sealers. During this period, differences were statistically significant (p=0.0002, Figure 2D) in the levels of nitrite produced by macrophages that were cultivated in contact with EPH and S26 (p<0.001) and EPH and ENF (p<0.01). At 48 h, ENF caused the J774/G8 cells to release less nitrite (p=0.0016, Figure 2E). There were significant differences between EPH and ENF (p<0.05) as well as between S26 and ENF (p<0.05). At 72 h, the average levels of nitrite were higher in EPH (p=0.0001, Figure 2F), withsignificant differences between EPH and AHP (p<0.001) and EPH and S26 (p<0.001).
Discussion
The complex response of periapical tissues that come into contact with sealant materials is the result of individual reactions of each cellular group involved as well as the reactions of the extracellular matrix. In this way, the present research aimed to evaluate the effect of four groups of freshly manipulated sealers on the viability of two types of cells over time as well as evaluate the release of nitric oxide, which is considered a cytotoxic mediator.
Immortalized cell cultures of fibroblasts and macrophages were evaluated for cytotoxicity in the periods of 24-72 h in order to closely mimic the initial cell response and simulate the worst scenario, wherein sealers are periapically extruded during obturation. Most in vitro studies use a fibroblast cell line (L929, Balb C 3T3, V79) to analyze the biological response to root canal sealers.4,6,8,10-11 Although macrophages are used less often, they represent the prevalent cells in inflammatory infiltrates that respond to the sealers12,17. They also play a key role in defense and repair by producing a myriad of substances with inflammatory activity12. The present study used two methods to evaluate cytotoxicity: cell viability through an MTT assay, an approach that has been widely used in many previous studies6,8,11,13,17 due to its simplicity, rapidity and reliability; and NO release by Griess reaction, which constitutes an important proinflammatory mediator13,15-16.
The cytotoxic responses of cells vary depending on the chemical composition of the sealer in a given experimental set-up. This study compared cell responses to freshly prepared resin-based, calcium hydroxide-based and zinc oxide eugenol-based sealers. Most products exert some toxic effect when they are fresh, and the effect lessens over time as the concentration of leachable components decreases10. The present study observed this same phenomenon in the fibroblasts cultures. Epiphany was the most cytotoxic of the sealers at 24 h. However, over time (72 h), Epiphany's toxic levels lowered to a similar pattern as the ones observed in the other 3 materials (p>0.05), with AH Plus having even smaller viability levels than Epiphany at 48h (p<0.05).
Similar to the present result, Susini et al., when using a root model, found that Epiphany was most cytotoxic at days 1 and 2, after which cytotoxicity decreased18. On the other hand, these results contradict previous studies, which found that Epiphany became more toxic with the time of exposure to cells4,19, while AH Plus had no or minimal cytotoxicity at 48h8. These conflicting results may be attributed to differences in the cell lines, as well as to the experimental design and methodology for cell viability analysis. Studies enrolling the cytotoxicity of endodontic sealer may differ regarding the use of fresh or set materials4, direct contact between sealers and cells, use of filter technique11 or Teflon molds to contain the sealers19, and treatment of cells with sealer extracts obtained after elution4,8.
In contrast to fibroblasts, the viability of macrophage cultures showed no statistically significant difference among the groups over time. The exception was between EPH and AHP at 72 h, with the former being more cytotoxic than the latter (p<0.05). This result suggests a late effect of toxic compounds, such as the residual monomers released by cured Epiphany on this cell line, occurring within the first 7 days of placement20. Another explanation for the cytotoxicity of Epiphany might be the leaching of the sealer's filler particles as a result of degradation8,10. Although the methodology used in this study does not allow researchers to identify which components are responsible for the cell response, the current result suggests that HFF may be more sensitive to Epiphany at initial periods than J774/G8 cells.
In relation to the production of nitrite by cells that were in contact with the sealers, a trend of increased production of this mediator was observed within the fibroblasts in contact with Epiphany. This pattern was truly evident at 72 h. These results indicate that the components of Epiphany activate a pro-inflammatory cascade that involves nitric oxide. Although the present study did not evaluate the influence of other mediators, such as TNFá and IL-1â21, on regulation of NO release, it is possible that those mediators will increase upon contact with this material. This could be related in vivo to an increase in the initial inflammatory reaction after inadvertent leakage that could occur during filling.
In the macrophage culture, the pattern of NO release was different from the pattern that was observed in the fibroblasts cultures. Different sealers changed this secretion profile over time. However, it is possible to observe high levels of NO produced by cells in contact with Epiphany for 24 h and 72 h. Contrary to previous studies17,22, which observed several toxic alterations to materials containing zinc oxide eugenol, the present research found that EndoFill presented the smallest absorbance levels of NO release at 48 h (p<0.05). EndoFill was also the least cytotoxic of the four sealers on macrophage cultures at initial times. However, in agreement with the present study, Queiroz et al.15 found that the NO levels were significantly smaller for EndoFill than for Sealer 26 at 48 h, possibly due to the components from the epoxy resin and formaldehyde release during the polymerization of the latter5.
Reports using relatively short-term in vitro tests (d"72 h) suggest that newer materials have varying degrees of initial cytotoxicity depending on the testing conditions6,11,19. A major limitation of current in vitro data is that the shortterm evaluations are likely to be inadequate for the prediction of biological responses of materials that remain in direct contact with periradicular tissues for as long as decades23. Recently, some investigators have extended in vitro test intervals to better simulate clinical conditions by using in vitro "aging" of the materials23. The results of these studies showed that many endodontic sealers remain severely cytotoxic for 5 or 6 weeks after they were mixed, but longerterm responses to other materials are unknown.
The results of this study raise a question about cytotoxicity and the induction of release of proinflammatory mediators by Epiphany. Conversely, the results in literature suggest different responses, which this study confirmed by using two different cell lines. More studies that use primary cell cultures and co-cultures should be conducted in order to evaluate these sealers in the short-, medium-, and long-term, as well as when sealers are newly prepared under fresh conditions. Thus, it would approximate to what is observed in vivo and it would be possible to compare the observations to those findings obtained within immortalized cultures.
The findings of the present study showed that the pattern of response to each sealer varied depending on time6,24 and cell line6,25, as previously demonstrated. Souza et al.25 reported that CFU-GM, which are macrophage progenitor cells, were more sensitive to endodontic sealers than fibroblast line. In general, the cytotoxic effects of root canal sealers may be considerably less intense over time6. However, some materials present a persistent inflammatory reaction after setting periods26. Although this study has only evaluated the toxicity of compounds released by fresh sealer (unset), the major cytotoxicity for EPH in short-term tests (<72h) deserves consideration. Accordingly, further in vitro and in vivo studies must be carried out for a more detailed evaluation of EPH behavior compared with other traditional endodontic sealers.
Acknowledgements
This research was supported by FAPEMIG.
References
1. Figueiredo JAP, Pesce HF, Gioso MA, Figueiredo MAZ. The histological effects of four endodontic sealers implanted in the oral mucosa: submucous injection versus implant in polyethylene tubes. Int Endod J. 2001; 34: 377-85. [ Links ]
2. Marciano MA, Guimarães BM, Ordinola-Zapata R, Bramante CM, Cavenago BC, Garcia RB, et al. Physical properties and interfacial adaptation of three epoxy resin-based sealers. J Endod. 2011; 37: 1417-21. [ Links ]
3. Cecchin D, Souza M, Carlini-Júnior B, Barbizam JV. Bond strength of Resilon/Epiphany compared with Gutta-percha and sealers Sealer 26 and Endo Fill. Aust Endod J. 2012; 38: 21-5. [ Links ]
4. Karapinar-Kazandag M, Bayrak ÖF, Yalvaç ME, Ersev H, Tanalp J, Sahin F, et al. Cytotoxicity of 5 endodontic sealers on L929 cell line and human dental pulp cells. Int Endod J. 2011; 44:626-34. [ Links ]
5. Queiroz CES, Soares JA, Leonardo RT, Carlos IZ, Dinelli W. Evaluation of cytotoxicity of two endodontic cements in a macrophage culture. J Appl Oral Sci. 2005; 13: 237-42. [ Links ]
6. Brackett MG, Messer RLW, Lockwood PE, Bryan TE, Lewis JB, Bouillaguet S, et al. Cytotoxic response of three cell lines exposed in vitro to dental endodontic sealers. J Biomed Mater Res B Appl Biomater. 2010; 95: 380-6. [ Links ]
7. Dahl JE. Toxicity of endodontic filling materials. Endod Topics. 2005; 12: 39-43. [ Links ]
8. Al-Hiyasat AS, Tayyar M, Darmani H. Cytotoxicity evaluation of various resin based root canal sealers. Int Endod J. 2010; 43: 148-53. [ Links ]
9. Bin CV, Valera MC, Camargo SE, Rabelo SB, Silva GO, Balducci I, Camargo CH. Cytotoxicity and genotoxicity of root canal sealers based on mineral trioxide aggregate. J Endod. 2012; 38: 495-500. [ Links ]
10. Eldeniz AU, Mustafa K, Orstavik D, Dahl JE. Cytotoxicity of new resin- , calcium hydroxide- and silicone-based root canal sealers on fibroblasts derived from human gingiva and L929 cell lines. Int Endod J. 2007; 40: 329-37. [ Links ]
11. Lodiene G, Morisbak E, Bruzell E, Orstavik D. Toxicity evaluation of root canal sealers in vitro. Int Endod J. 2008; 41: 72-7. [ Links ]
12. Mendes STO, Sobrinho APR, Carvalho AT, Côrtes MIS, Vieira LQ. In vitro evaluation of the cytotoxicity of two root canal sealers on macrophage activity. J Endod. 2003; 29: 95-9. [ Links ]
13. da Silva PT, Pappen FG, Souza EM, Dias JE, Bonetti Filho I, Carlos IZ, et al. Cytotoxicity evaluation of four endodontic sealers. Braz Dent J. 2008; 19: 228-31. [ Links ]
14. Soares JA, Queiroz CES. Patogenesia periapical - aspectos clínicos, radiográficos e tratamento da reabsorção óssea e radicular de origem endodôntica. JBE. 2001; 2: 124-35. [ Links ]
15. Queiroz CE, Soares JA, Leonardo Rde T, Carlos IZ, Dinelli W. Evaluation of cytotoxicity of two endodontic cements in a macrophage culture. J Appl Oral Sci. 2005; 13: 237-42. [ Links ]
16. Takeichi O, Saito I, Hayashio M, Tsurumachi T, Saito T. Production of human-inducible nitric oxide synthase in radicular cysts. J Endod. 1998; 24: 157-60. [ Links ]
17. Silva PT, Leonardo RT, Carlos IZ, Bonetti-Filho I. Avaliação da citotoxicidade de cimentos endodônticos em relação aos reativos intermediários do oxigênio e do nitrogênio em culturas de macrófagos peritoneais de camundongos. Rev Odontol UNESP. 2005; 34: 17-23. [ Links ]
18. Susini G, About I, Tran-Hung L, Camps J. Cytotoxicity of Epiphany and Resilon with a root model. Int Endod J. 2006; 39: 940-4. [ Links ]
19. Bouillaguet S, Wataha JC, Tay FR, Brackett MG, Lockwood PE. Initial in vitro biological response to contemporary endodontic sealers. J Endod. 2006; 32: 989-92. [ Links ]
20. Ruyter IE. Physical and chemical aspects related to substances released from polymer materials in an aqueous environment. Adv Dent Res. 1995; 9: 344-7. [ Links ]
21. Bose M, Farnia P. Proinflammatory cytokines can significantly induce human mononuclear phagocytes to produce nitric oxide by a cell maturation dependent process. Immunol Lett. 1995; 48: 59-64. [ Links ]
22. Schwarze T, Leyhausen G, Geurtsen W. Long-term cytocompatibility of various endodontic sealers using a new root canal model. J Endod. 2002; 28: 749-53. [ Links ]
23. Brackett MG, Marshall A, Lockwood PE, Lewis JB, Messer RL, Bouillaguet S, et al. Cytotoxicity of endodontic materials over 6-weeks ex vivo. Int Endod J. 2008; 41: 1072-8. [ Links ]
24. Ma J, Shen Y, Stojicic S, Haapasalo M. Biocompatibility of two novel root repair material. J Endod. 2011; 37: 793-8. [ Links ]
25. Souza NJA, Justo GZ, Oliveira CR, Haun M, Bincolleto C. Cytotoxicity of materials used in perforation repair tested using the V79 fibroblast cell lines and the granulocyte-macrophage progenitor cells. Int Endod J. 2006; 39: 40-7. [ Links ]
26. Brackett MG, Marshall A, Lockwood PE, Lewis JB, Messer RL, Bouillaguet S, et al. Inflammatory suppression by endodontic sealers after aging 12 weeks in vitro. J Biomed Mater Res Part B: Appl Biomater. 2009; 91: 839-44. [ Links ]
 Correspondence:
Correspondence:
Camilla Christian Gomes Moura
Departamento de Endodontia - Faculdade de
Odontologia da Universidade Federal de Uberlândia
Av. Pará 1720 - Bloco 2B - Jardim Umuarama
CEP: 38400-902 - Uberlândia, MG - Brasil
E-mail: camillahistologia@yahoo.com.br
Received for publication: April 02, 2012
Accepted: June 19, 2012













