Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.11 no.3 Piracicaba Jul./Set. 2012
ORIGINAL ARTICLE
Effect of cetylpyridinium chloride with xylitol on the formation of biofilm and development of gingivitis
Bruna GhiraldiniI; Erika Tie FurushimaII; Renato Corrêa Viana CasarinIII; Karina Teixeira VillalpandoIV; Suzana Peres PimentelIII; Fabiano Ribeiro CiranoIII
IDentist, UNIP, São Paulo, SP, Brazil
IIUndergraduate student, UNIP, São Paulo, SP, Brazil
IIIMsC, PhD, Professor of Periodontics, UNIP, São Paulo, SP, Brazil
IVMsC, PhD, Professor of Periodontics and Dental Clinic, PUC, Campinas, SP, Brazil
ABSTRACT
AIM: To assess the effect of the combination of cetylpyridinium chloride and xylitol on the formation of dental biofilm and development of experimental gingivitis.
METHODS: A crossover, double-blind, placebo-controlled study was conducted and divided into two phases of 21 days each with a time interval of 10 days between them. A modified experimental gingivitis model was used and 31 volunteers were randomly divided into 2 groups. The volunteers performed daily mouthwashes twice a day with the test solution containing cetylpyridinium combined with xylitol or a placebo solution. On day 0 and day 21 of each phase the Plaque Index (PI) and Gingival Index (GI) of each volunteer were measured. During this phase, the volunteers brushed their teeth with standard toothbrushes and dentifrice, protecting the third quadrant with a toothshield. After brushing, the toothshield was removed and the mouthwash was used.
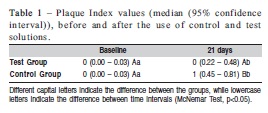
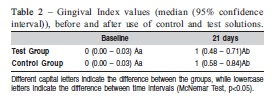
RESULTS: The PI values observed in the Test Group at baseline and on day 21 were 0 (0.00 – 0.03) and 0 (0.22-0.48) respectively, and in Control Group 0 (0.00 – 0.03) and 1 (0.45 – 0.81) (inter-group analysis - McNemar test, p<0.05). For GI, the values obtained in the Test Group were 0 (0.00 – 0.03) and 1 (0.48 – 0.71), at baseline and day 21 and in Control Group 0 (0.00 – 0.03) and 1 (0.58 – 0.84) (inter-group analysis - McNemar test, p>0.05).
CONCLUSIONS: The test solution had a positive effect on dental biofilm control. However, it was not capable of preventing the development of experimental gingivitis.
Keywords: cetylpyridinium chloride, xylitol, gingivitis.
Introduction
Mechanical control of dental biofilm is an important factor for preventing gingival inflammation and dental caries. However, the daily use of a toothbrush and interdental cleaning devices are not adequately performed by most individuals. Therefore, the use of substances with a potential of chemical control of dental biofilm may be indicated1-2. In this connection, mouthwashes are frequently recommended as well as several products containing different active ingredients.Among the different types of substances, two cationic antiseptics called chlorhexidine and cetylpyridinium chloride (CPC) deserve to be pointed out. With a view to reducing the quantity and virulence of biofilm, these substances promote a reduction in the inflammatory response3.
Chlorhexidine has been considered the gold standard regarding chemical control of dental biofilm, presenting the highest values of plaque reduction within oral antiseptics (plaque reduction of 58.3% to 92.9%)4-5. However, chlorhexidine causes pigmentation of teeth and restorations, has an unpleasant flavor, leads to taste alterations, increases the formation of supragingival calculus and, it may be associated with mucosal desquamation. Therefore, the longterm daily use of chlorhexidine is not recommended and alternative substances that present efficacy in biofilm control and reduced adverse effects could be considered an important therapeutic approach. CPC is capable of reducing biofilm formation4-12, reducing plaque index 34.5% to 70.9%13, and although presents same side effects, such as dental pigmentation, they are much less intense than chlorhexidine4-13.
Although the use of CPC has been shown to be a feasible option as an adjuvant in controlling biofilm, its potential could be increased by the association of other substances that could contribute towards this purpose. In this regard, xylitol has shown to be effective in preventing dental caries. Clinical studies that combined xylitol with the use of fluoridated dentifrices, dietary and behavioral changes have shown the efficacy of this concomitant therapy5,14.
Studies have indicated that xylitol might act in the reduction of caries incidence15 by decreasing the number of Streptococcus mutans with its prolonged use16, indicating that xylitol might decrease the ability of bacteria to multiply in its presence. Furthermore, Hildebrant and Sparks17 (2000) showed that chlorhexidine mouthwash reduces S. mutans levels and long-term use of xylitol is capable of maintaining these levels low.
In view of the above, the aim of this study was to evaluate the effect of a CPC and xylitol solution on supragingival plaque formation and development of experimental gingivitis.
Material and methods
Thirty-one volunteers participated in this study. The research subjects were selected after signing an informed consent form and the research was approved by the Research Ethics Committee of the Paulista University under protocol #492/09. The following inclusion criteria were adopted: be in the age group between 18 and 28 years, have no medical history of systemic diseases, and have at least 20 teeth in the mouth. The following exclusion criteria were considered: be a smoker, have used systemic antibiotics in the 3 months previous to the study, have used chemical agents to control plaque 15 days before the study, be allergic to CPC and/or xylitol, be pregnant, be a permanent drug user, wear prosthesis or orthodontic appliances, and have probing depth greater than 3 mm.
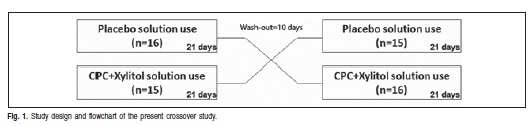
A crossover, double-blind, placebo-controlled study was conducted in accordance with the modified experimental gingivitis model. The volunteers were randomly divided into two groups: Test Group (0.5 % CPC + 12.5% xylitol solution) and Control Group (placebo) and the study consisted of an experimental phase composed of two periods of 21 days, with intervals of 10 days between them (wash out period).
Impressions were made of the left mandibular hemi-arch of the volunteers with alginate and the resulting model was used for preparation of a toothshield using polyvinyl acetate lamina prepared in a vacuum plasticizer and cut to cover the entire area of teeth 34, 35, 36 and 37 and 2 mm beyond the gingival margin both in the vestibular and lingual surfaces, which was used until the end of the experiment.
Samples containing CPC combined with xylitol (Atco Pharma, São Paulo, SP, Brazil) and a placebo agent (solution with the same flavor and coloring of the test solution, but with no CPC or xylitol) were given to the volunteers in identical bottles so that neither the examiner nor the volunteer could identify them. Both the test and placebo solutions were properly codified and the secrecy of the codes was revealed only at the end of the study. All the participants tested the chemical agent in alternating periods in accordance with the proposed crossover study.
Before the beginning of each study period, professional removal of the supragingival dental biofilm was performed. Afterwards, during each period of 21 days, the volunteers performed normal oral hygiene using the acetate toothshield so that the area selected did not receive mechanical control of biofilm. Each research subject was instructed to use a standard toothbrush and dentifrice (Professional Colgate toothbrush and MFP Colgate dentifrice, Colgate-Palmolive, São Bernardo do Campo, SP, Brazil), and the dentifrice used did not have any active ingredient besides fluoride. Twice a day (every 12 h) the volunteers performed mouthwashing with 20 mL of the determined solution for 1 min, without acetate toothshield18-19.
The bottles of the solutions used and the ones that were not used were returned at the end of each period to prevent them from being reused. New bottles with the new solution were provided after the time interval of 10 days, which means that the group that used the test solution started using the placebo solution and the other group the opposite (Figure 1). The toothshields were assessed by the researcher and replaced when damaged and the reusable ones were washed and disinfected between the experimental periods. In the washout period the volunteers practiced conventional oral hygiene with a dentifrice and toothbrush in the entire oral cavity.
The assessments of the clinical parameters performed on day 0 and day 21 of each period were dichotomously done, at six sites per tooth, at teeth covered by toothshield, using visible plaque20 and gingival indices21.
The indexes used were codified as follows: Plaque Index (PI): 0 = absence of plaque and 1 = presence of plaque; Gingival index (GI): 0 = absence of bleeding on probing and 1 = presence of bleeding on probing.
All clinical examinations were performed by a previously calibrated clinician (BG – Kappa Index = 0.85). For statistical analysis, the non-parametric McNemar test was used and a level of significance of 5% was adopted.
Results
All the volunteers (65% female, mean age 21.1±2.2) accepted the research conditions in a satisfactory manner and they all completed the study until the end. It was observed a statistically significant increase in the PI in the Test and Control Group (Table 1). However, inter-group analysis showed higher PI in the Control Group (p<0.05). In addition, an increase in GI in the two groups (p<0.05) was observed. Nevertheless, in the inter-group analysis no statistically significant differences were observed on day 21 (Table 2).
Discussion
As biofilm is considered the primary etiologic factor of periodontal diseases, prevention approached focus on different forms to inhibit its formation and development. In this effort, mechanical control and chemical agents, alone or in association has been used for obtain periodontal health. Within several chemical agents, Chlorhexidine is considered the gold standard, although it has very intense side effects. Thus, CPC appears as an alternative agent, present antibacterial action and lesser side effects. Moreover, some agents could be added to CPC solution, promoting other oral benefits. Recently, a combination of CPC and xylitol, a recognized anti-caries agent, has been produced, but its effect on plaque and gingivitis control is yet unknown. Therefore, the present study was conducted to analyze the effect of a CPC and xylitol solution on the formation of supragingival biofilm and development of experimental gingivitis in comparison with a placebo solution.
The findings of the present crossover randomized study indicate that CPC+xylitol mouthwash has potential in controlling supragingival dental biofilm, as inter-group differences were seen regarding plaque accumulation after the experiment. However, the potential of this mouthwash in controlling dental biofilm did not result in benefits on the development of experimental gingivitis. Comparing the bleeding on probing after use of CPC+xylitol and placebo solutions, no inter-group differences were observed (p<0.05). These results indicate that the CPC+xylitol solution has a significant benefit on plaque control, although it did not lead to benefits in controlling the development of gingivitis.
Similar results were observed by several studies that showed a significant reduction in PI when CPC was used in comparison with a placebo solution5,7-10,12,22-24. The antiplaque effect of CPC is due to its ability to decrease the surface tension of water and alter bacterial cell permeability that causes the output of enzymes and essential metabolites25. This action allows CPC to penetrate the bacterial cell membrane causing destruction of cellular components, disruption of bacterial metabolism and inhibition of cell growth, ultimately leading to cell death and its interference with bacterial adherence26. Albert-Kiszely et al.26 (2007) found that its use, in the form of mouthwash, caused a reduction in the number of bacteria adhered to epithelial cells of the oral mucosa.
The present study showed that the test solution was not capable of promoting actual beneficial effects in GI, despite having promoted a statistically significant reduction in PI. However, Ayad et al.9 (2011) and Silva et al.24 (2009) found a statistically significant difference in GI, showing that CPC was superior in comparison with the placebo solution. It is worth mentioning that both studies had different methodologies from that of the present study because they did not use the modified experimental gingivitis model2, the research subjects used chemical control in combination with mechanical control, and longer evaluation periods, namely 6 weeks in the study of Silva et al.24 (2009), and 3 and 6 months in the study of Ayad et al.9 (2011). In the other hand, Rioboo et al.27 (2012) showed limited benefits of the CPC as adjuncts to unsupervised oral hygiene in reducing plaque accumulation, and no effect on gingivitis, corroborating to our results.
Clinical studies have shown that the combination of xylitol with the use of fluoridated dentifrices and dietary and behavioral changes reduces dental biofilm and cariogenic bacteria14. This action may be the result of the decrease in the number of S. mutans with the use of the substance or the potential of xylitol in penetrating the biofilm by diffusion and reducing adhesiveness of the bacteria16,28-29. Perhaps these characteristics of xylitol may contribute to explain the results of the present study, which are in agreement with other studies that have shown the potential for reducing dental biofilm30.
The results of this study showed the potential of the combination of CPC and xylitol in preventing the formation of dental biofilm, but these are short-term results and with no mechanical control. It is important to consider that this model of experimental gingivitis allows evaluating the effect of chemical agents on plaque formation and gingivitis development, removing the effect of mechanical control. In our opinion, this represents one of the best forms to determine the efficacy of mouthwash alone. However, sometimes, it did not represent the usual clinical condition and, further long-term studies combining the solution with mechanical control should be made seeking greater effectiveness in controlling gingival inflammation.
Within the limitations of this study, it may be concluded that the combination of CPC and xylitol has the potential to control the formation of dental biofilm, but it does not have any effect on the development of experimental gingivitis.
References
1. Hull PS. Chemical inhibition of plaque. J Periodontol. 1980; 7: 431-42. [ Links ]
2. Lindhe J, Koch G. The effect of supervised oral hygiene on the gingivae of children. J Periodontol. 1967; 2: 215-20. [ Links ]
3. Axelsson P, Albandar JM, Rams TE. Prevention and control of periodontal diseases in developing and industrialized nations. Periodontol 2000. 2002; 29: 235-46. [ Links ]
4. Quirynen M, Soers C, Desnyder M, Dekeyser C, Pauwels M, Van Steenberghe D. A 0.05% cetylpyridinium chloride/0.05% chlorhexidine mouth rinse during maintenance phase after initial periodontal therapy. J Clin Periodontol. 2005; 32: 390-400. [ Links ]
5. Paula VA, Modesto A, Santos KR, Gleiser R. Antimicrobial effects of the combination of chlorhexidine and xylitol. Br Dent J. 2010 Dec 18; 209: E19. [ Links ]
6. Witt JJ, Walters P, Bsoul S, Gibb R, Dunavent J, Putt M. Comparative clinical trial of two antigingivitis mouthrinses. Am J Dent. 2005; 18: 15A-7A. [ Links ]
7. Charles CA, McGuire JA, Sharma NC, Qaqish J. Comparative efficacy of two daily use mouthrinses: randomized clinical trial using an experimental gingivitis model. Braz Oral Res. 2011; 25: 338-44. [ Links ]
8. García V, Rioboo M, Serrano J, O'Connor A, Herrera D, Sanz M. Plaque inhibitory effect of a 0.05% cetyl-pyridinium chloride mouth-rinse in a 4-day non-brushing model. Int J Dent Hyg. 2011; 9: 266-73. [ Links ]
9. Ayad F, Prado R, Mateo LR, Stewart B, Szewczyk G, Arvanitidou E et al. A comparative investigation to evaluate the clinical efficacy of an alcoholfree CPC-containing mouthwash as compared to a control mouthwash in controlling dental plaque and gingivitis: a six-month clinical study on adults in San Jose, Costa Rica. J Clin Dent. 2011; 22: 204-12. [ Links ]
10. Rao D, Arvanitidou E, Du-Thumm L, Rickard AH. Efficacy of an alcoholfree CPC containing mouthwash against oral multispecies biofilms. J Clin Dent. 2011; 22: 187-94. [ Links ]
11. Williams MI. The antibacterial and antiplaque effectiveness of mouthwashes containing cetylpyridinium chloride with and without alcohol in improving gingival health. J Clin Dent. 2011; 22: 179-82. [ Links ]
12. Barnes VM, Arvanitidou E, Szewczyk G, Richter R, DeVizio W, Cronin M et al. Evaluation of the antiplaque efficacy of two cetylpyridinium chloridecontaining mouthwashes. J Clin Dent. 2011; 22: 200-3. [ Links ]
13. He S, Wei Y, Fan X, Hu D, Sreenivasan PK. A clinical study to assess the 12-hour antimicrobial effects of cetylpyridinium chloride mouthwashes on supragingival plaque bacteria. J Clin Dent. 2011; 22: 195-9. [ Links ]
14. Takahashi N, Washio J. Metabolomic effects of xylitol and fluoride on plaque biofilm in vivo. J Dent Res. 2011; 90: 1463-8. [ Links ]
15. Macek MD. Xylitol-Based Candies and Lozenges may Reduce Caries on Permanent Teeth. J Evid Based Dent Pract. 2012; 12: 71-3. [ Links ]
16. Tanzer JM. Xylitol chewing gum and dental caries. Int Dent J. 1995; 45: 65-76. [ Links ]
17. Hildebrant GH, Sparks BS. Maintaining mutans streptococci suppression with xylitol chewing gum. J Am Dent Assoc. 2002; 131: 909-16. [ Links ]
18. Nogueira-Filho GR, Toledo S, Cury JA. Effect of 3 dentifrices containing triclosan and various additives. An experimental gingivitis study. J Clin Priodontol. 2000; 27: 494-8. [ Links ]
19. Nogueira-Filho GR, Duarte PM, Toledo S, Tabchoury CP, Cury JA. Effect of triclosan dentifrices on mouth volatile sulphur compounds and dental plaque trypsin-like activity during experimental gingivitis development. J Clin Periodontol. 2002; 29: 1059-64. [ Links ]
20. Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975; 25: 229-35. [ Links ]
21. Mühlemann HR, Son S. The effect of human supragingival calculus formation of acetohydroxamic acid. Helv Odontol Acta. 1971; 15: suppl 7: 158-9. [ Links ]
22. Lotufo R, Calil CM, Feng HS, Sekiguchi RT, Stewart B, De Vizio W et al. Clinical investigation of a commercial mouthrinse containing 0. 05% cetylpyridinium chloride in preventing dental plaque. J Clin Dent. 2009; 20: 50-4. [ Links ]
23. Hernandez-Cott PL, Boneta AE, Stewart B, DeVizio W, Proskin HM. Clinical investigation of the efficacy of a commercial mouthrinse containing 0.05% cetylpyridinium chloride in reducing dental plaque. J Clin Dent. 2009; 20: 39-44. [ Links ]
24. Silva MFA, Santos NB, Stewart B, De Vizio W, Proskin HM. A clinical investigation of the efficacy of a commercial mouthrinse containing 0.05% cetylpyridinium chloride to control established dental plaque and gingivitis. J Clin Dent. 2009; 20: 55-61. [ Links ]
25. Rane VS, Davison G, Borutell P, Gallitschke N. 1092 changes in gumline plaque pathogenicity: 3 weeks CPC treatment. J Dent Res. 2006; A: 626. [ Links ]
26. Albert-Kszely A, Pjetursson BE, Salvi GE, Witt J, Hamilton A, Persson GR et al. Comparison of the effects of cetylpyridinium chloride with an essential oil mouth rinse on dental plaque and gingivitis – a six – month randomized controlled clinical trial. J Clin Periodontol. 2007; 34: 658-67.
27. Rioboo M, García V, Serrano J, O'Connor A, Herrera D, Sanz M. Clinical and microbiological efficacy of an antimicrobial mouth rinse containing 0.05% cetylpyridinium chloride in patients with gingivitis. Int J Dent Hyg. 2012; 10: 98-106. [ Links ]
28. Maguire A, Rugg-Gunn AJ. Xylitol and caries prevention – is it a magic bullet?. Br Dent J. 2003; 194: 429-36.
29. Söderling EM. Xylitol, Mutans streptococci, and dental plaque. Adv Dent Res. 2009; 21: 74-8. [ Links ]
30. Badet C, Furinga A, Thebaud N. Effect of xylitol on an in vitro model of oral biofilm. Oral Health Prev Dent. 2008; 6: 337-41. [ Links ]
 Correspondence:
Correspondence:
Fabiano Ribeiro Cirano
Professor of Periodontics,
Universidade Paulista (UNIP)
Rua Dr. Bacelar, 1212 - CEP: 04026-002
Vila Clementino, São Paulo, SP - Brasil
E-mail: cirano@unip.br
Received for publication: June 26, 2012
Accepted: September 18, 2012













