Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.11 no.3 Piracicaba Jul./Set. 2012
ORIGINAL ARTICLE
Influence of two methods of additional activation on composite resins surface hardness
Alessandro Ribeiro GonçalvesI; Caroline de Deus Tupinambá RodriguesII; Carlos Henrique de Carvalho e SouzaIII; Leilane Ferraz Moreira de SousaIV; Pedro Henrique de Souza LopesIV
IPhD in Oral Rehabilitation – FOAR/UNESP, Assistant Professor, Department of Restorative Dentistry, Federal University of Piauí (UFPI), Teresina, PI, Brazil
IIPhD in Restorative Dentistry – FOAR/UNESP, Assistant Professor, Department of Restorative Dentistry, Federal University of Piauí (UFPI), Teresina, PI, Brazil
IIIDentistry Master's Student, Department of Restorative Dentistry, Federal University of Piauí (UFPI), Teresina, PI, Brazil
IVScientific Initiation Student, Department of Restorative Dentistry, Federal University of Piauí (UFPI), Teresina, PI, Brazil
ABSTRACT
AIM: To evaluate the influence of two methods of additional activation on the surface hardness of composite resins.
METHODS: Two types of composites were tested: Filtek P60 and Filtek P350. For each material, 48 specimens were prepared and divided into four groups: Group 1 (control) - conventional activation, using a halogen light for 40 s; Group 2 - conventional activation and additional activation with a halogen lamp for 60 s; Group 3 - conventional activation and additional activation with an autoclave at 127°C for 6 min at 1.7 kg/cm3 pressure; and Group 4 - conventional activation and additional activation with an autoclave at 134 °C for 15 min at 2.1 kg /cm3 pressure. The use of autoclave has been suggested for being a standard equipment at dental offices, and thus, even at locations far from dental laboratories, it would be possible to have simple techniques that allow access to indirect restorations at lower costs. Data obtained in the study were analyzed statistically by analysis of variance followed by Tukey's test at a 5% level of significance.
RESULTS: For Z350, there was a significant increase in hardness for all groups of additional activation (Groups 2, 3 and 4), compared with the control group. For P60, a significant increase in surface hardness was found compared with the control group for the groups that used additional activation with an autoclave (Groups 3 and 4).
CONCLUSIONS: Additional activation with an autoclave increased the surface hardness of the tested resins to a greater degree than additional activation with a halogen light.
Keywords: polymerization, composite resins, hardness.
Introduction
Technological evolution in dentistry is driven by the constant attempt to improve materials and techniques in line with the market's demands. Such advances mean that it is increasingly possible for clinicians to produce outcomes that combine function with good aesthetics. At the moment, the most widely used restorative materials in dentistry are composite resins, mainly because they adhere well to the tooth structure, have suitable mechanical properties and are available in a wide range of shades and translucencies that produce good aesthetic results1.
Since their introduction as restorative materials for posterior teeth, composite resins have significantly improved in terms of their physical and mechanical characteristics. The composition of their organic and inorganic matrix has changed, and there are currently various types of resins that can be used in posterior teeth, such as microhybrid resins and composites with nanoparticles2-3. Composite resins are usually recommended for direct restorations in posterior teeth when cavities are small and medium in size. To restore large cavities and extensively destroyed teeth, dentists generally opt for amalgam fillings or indirect laboratory-made resin, porcelain or metallic restorations4-5. Amalgam can be used for direct restorations, but it is limited by its unaesthetic outcome. The main limiting factor of indirect restorations is their high cost.
One viable alternative (published in the norms of the manufacturers of resins indicated for use in posterior teeth) is the use of these resins in indirect restorations such as crowns, veneers, inlays, onlays and so on. However, these indirect restorations require additional costs and special equipment for activation. Various studies have shown that by means of simple technical changes, such as additional activation, direct-use resins can achieve mechanical properties that are similar to laboratory-made restorations6-8.
Different methods of extraoral additional activation, including activation by light, dry heat and autoclaving, were proposed in order to improve physical and mechanical properties, and enable the use of direct-use composite resins in indirect restorations. The purpose of this additional treatment is to broaden the indication of resins and the clinical longevity of restorations7-9. However, there are few studies that investigate which of these are the best method. The autoclave has been suggested because it is a standard equipment at dental offices. Therefore, even at locations far from dental laboratories, it would be possible to develop simple techniques that could allow access to indirect restorations at lower costs.
The surface hardness of composite resins depends mainly on their microstructure and composition, but there is a correlation with the degree of conversion of monomers2-10. The increase in hardness is related to a higher degree of conversion and improved mechanical properties of polymeric materials. Thus, the study of the microhardness of materials consists of an indirect method to evaluate the effect of different treatments on the properties of dental composites10-12. The Vickers hardness test is commonly used to investigate improvement in material's mechanical properties2,13-16. Using this test as a parameter, the aim of this study was to evaluate the microhardness of two direct resin composites after additional activation with light or using an autoclave.
Material and methods
Specimen preparation
To obtain the resin specimens, a circular split-ring matrix with an outer diameter of 2 cm and an inner diameter of 1 cm was used. It was held in position by a circular metal matrix with a 2-cm diameter perforation and 3-cm outer diameter (Figure 1 and 2).
The composite resins used were: 1) Filtek P60 (3M ESPE, St. Paul, MN, USA) – a hybrid composite, for direct and indirect restorations in posterior teeth and 2) Filtek Z350 (3M ESPE) – a nanoparticulate composite, for direct anterior and posterior restorations and for indirect inlay, onlay and veneer restorations (Table 1). P60 was used in this study to an extensively researched material, with a large number of published papers, and because it is a material with an indication for use in posterior teeth, region usually requires partial indirect restorations. Z350 composite was selected due to its recent launch in the dental market and for representing the newest class of composite resins with an indication for anterior and posterior teeth.
The matrix was placed on a glass plate and the inner space was filled with one of the studied composite resins in a single increment. A polyester strip was placed on the top surface and activated for 60 s using a Kondertech activation device, model CL-K200 (Kondortech, São Carlos, SP, Brazil) having a light intensity of 500 mW/cm2. The light intensity was checked with a digital radiometer (Dabi-Atlante, São Paulo, SP, Brazil). Ninety-six specimens were prepared, 48 for each restorative material, divided into the following groups: Group 1 (control) – conventional activation, using a halogen light for 60 s; Group 2 – conventional activation and additional activation with a halogen light for an additional 60 s; Group 3 – conventional activation and additional activation with an autoclave at 127°C for 6 min and 1.7 kg/cm3 pressure; Group 4 – conventional activation and additional activation with an autoclave at 134°C for 15 mins at 2.1 kg/cm3 pressure.
Hardness Test
After they had been prepared, the specimens were stored in distilled water in a bacteriological oven at 37°C for 7 days in a light proof container. The Vickers microhardness tests were carried out with the aid of a MMT-3 Microhardness Tester (Buehler, Lake Bluff, IL, USA) under a load of 50 gF for 30 s. Three microhardness impressions were carried out per sample, one in the center and two at the periphery, as follows: traced an imaginary line dividing the sample in half, a central impression was made and the other between the center and right and left edges.
Statistical analysis
The data obtained from the means were subjected to analysis of variance (ANOVA) followed by Tukey's test at a 5% level of significance.
Results
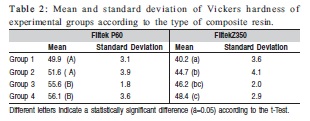
The tested resins had different means of hardness, according to the type of material and the type of treatment of the samples, as shown in Table 2.
Discussion
The results of this study indicate that additional activation methods significantly improved the microhardness of the composite resins evaluated. These results are consistent with the study of Dickerson and Hastings17 (1995), which reported polymerization rates of approximately 50% to 60% for self-activated resins and 55% to 65% for photoactivated resins, and reported that these resins reached a degree of conversion of 80-85% when subjected to a temperature of 125°C.
The use of an autoclave for additional activation (groups 3 and 4) significantly increased the microhardness compared with the control group, for both materials tested, probably because the autoclave generates greater amounts of energy in the form of heat and pressure, which increases the conversion rate. Other studies that evaluated the influence of heat treatment also found improvements in the mechanical properties of materials, which may be related to a reduction in the amount of residual double carbon bonds in the polymer18-21.
In another study of the behavior of resins and additional activation, Bagis and Rueggeberg6 (2000) reported that heat treatment increases the conversion rate, and this increase would not be possible if it was only photoactivated. Table 2 confirms this statement, and even the use of additional photoactivation time did not result in a greater hardness of Filtek P60.
Perhaps this difference is due to the composition of the resins. Trujillo, Newman and Stansbury22 (2004) reported that exposure of composites to additional heat treatment, limited to a biologically compatible time period, significantly affects the kinetics of activation, and increases the conversion rate of resins and improves their properties.
The increase in hardness can be explained by the fact that the temperatures used in the heat treatment were close to the glass transition temperature, which increases the kinetic energy of the resinous monomers and the quantity of free radicals. The greater mobility within the polymer chain enables new reactions of the activated radicals and a greater number of crosslinks in the organic matrix. The continuation of the activation process leads to greater stability and hardness of the composite7-8,21,23.
With Filtek Z350 resin, as well as the additional conversion using heat and pressure, the longer light exposure (exceeding the amount recommended by the manufacturer) affected significantly the surface hardness. This fact suggests that, although the microhardness of this material is suitable for dental needs, it is possible to produce a higher conversion of residual monomers through greater exposure to halogenlight and thereby increase the restoration's durability24.
Another advantage is the greater biocompatibility of the material. Increased activation results in a significant decrease in the amount of non-activated monomers and, consequently, lower levels of leachable materials that promote oral cytotoxicity6. The use of heat also causes a similar effect: it results in more monomers linked to the polymer chain and some of the unreacted monomers are volatilized during the heating process25.
The effect of additional activation and the different microhardness values of the resinous materials depend mainly on their composition. BIS-GMA has a low degree of conversion because of its characteristics of high molecular weight, high viscosity and low flexibility. The addition of diluent monomers with higher flexibility, such as EGDMA or TEGDMA, enhances Bis-GMA's mobility and its polymerization conversion rate. Another alternative to Bis- GMA is the monomer UDMA, which has a molecular weight similar to Bis-GMA, but a lower viscosity18,26.
It has been shown that the monomer TEGDMA creates a thicker polymer chain, but it is the most flexible and has a greater rate of water absorption. Bis-GMA forms a more rigid chain and absorbs less water; however, it absorbs more water than the UDMA/Bis-EMA combination. Hydrolysis of intermolecular bonds weakens the polymer. In UDMA-based composites, hydrogen bonds increase the conversion rate and improve mechanical properties. When TEGDMA is replaced by UDMA and/or BIS-EMA (during co-polymerization with BIS-GMA), the absorption of water is decreased. Such characteristics influence the conversion rate and the mechanical properties of composites27-28. The abovementioned information explains the different behaviors between the Z350 and P60 composites after additional activation: Z350 contains BIS-GMA, UDMA, TEGDMA and BIS-EMA, while P60 contains BIS-GMA, UDMA and BIS-EMA.
From the above discussion, it is possible to state that additional activation by means of thermal treatments improved the hardness of the composite resins tested, regardless of their composition. The use of an autoclave as an additional method of activation is very effective and provides better results. By using a routinely found equipment at dental offices, such as autoclave, it is possible to develop a simple, low cost technique, especially in cases of difficult access to special equipment for the production of laboratory restorations.
It can be concluded that the additional activation using an autoclave increased the surface hardness of the tested resins more than additional activation with halogen light.
References
1. Anfe TE, Caneppele TM, Agra CM, Vieira GF. Microhardness assessment of different commercial brands of resin composites with different degrees of translucence. Braz Oral Res. 2008; 22: 358-63. [ Links ]
2. Hosseinalipour M, Javadpour J, Rezaie H, Dadras T, Hayati AN. Investigation of mechanical properties of experimental Bis-GMA/TEGDMA dental composite resins containing various mass fractions of silica nanoparticles. J Prosthodont. 2010; 19: 112-7. [ Links ]
3. Monteiro GQM, Montes MAJR. Evaluation of linear polymerization shrinkage, flexural strength and modulus of elasticity of dental composites. Mat. Res. 2010; 13: 51-5. [ Links ]
4. Christensen GJ. Considering tooth-colored inlays and onlays versus crowns. J Am Dent Assoc. 2008; 139: 617-20. [ Links ]
5. Krämer N, García-Godoy F, Reinelt C, Feilzer AJ, Frankenberger R. Nanohybrid vs. fine hybrid composite in extended Class II cavities after six years. Dent Mater. 2011; 27: 455-64. [ Links ]
6. Bagis YH, Rueggeberg FA. The effect of post-cure heating on residual, unreacted monomer in a commercial resin composite. Dent Mater. 2000; 16: 244-7. [ Links ]
7. Lombardo GHL, Carvalho CF, Galhano G, Souza ROA, Nogueira Júnior L, Pavanelli CA. Influence of additional polymerization in the microhardness of direct composite resins. Cienc Odontol Bras. 2007; 10: 10-5. [ Links ]
8. Santana IL, Lodovici E, Matos JR, Medeiros IS, Miyazaki CL, Rodrigues-Filho LE. Effect of experimental heat treatment on mechanical properties of resin composites. Braz. Dent. J. 2009; 20: 205-10. [ Links ]
9. Coelho LFB, Herbstrith SRM, Mota EG, Oshima HMS, Balbinot CE, Bondan JL. Influence of different secondary cure techniques on hardness of composite resins. Rev Odonto Cienc. 2007; 22: 317-20. [ Links ]
10. Camargo EJ, Moreschi E, Baseggio W, Cury JA, Pascotto RC. Composite depth of cure using four activation techniques. J Appl Oral Sci. 2009; 17: 446-50. [ Links ]
11. Price RB, Fahey J, Felix CM. Knoop hardness of five composites cured with single-peak and polywave LED curing lights. Quintessence Int. 2010; 41: e181-91. [ Links ]
12. Albino LG, Rodrigues JA, Kawano Y, Cassoni A. Knoop microhardness and FT-Raman evaluation of composite resins: influence of opacity and photoactivation source. Braz Oral Res. 2011; 25: 267-73. [ Links ]
13. Bhamra GS, Fleming GJ, Darvell BW. Influence of LED irradiance on flexural properties and Vickers hardness of resin-based composite materials. Dent Mater. 2010; 26: 148-55. [ Links ]
14. Ceballos L, Fuentes MV, Tafalla H, Martínez A, Flores J, Rodríguez J. Curing effectiveness of resin composites at different exposure times using LED and halogen units. Med Oral Patol Oral Cir Bucal. 2009; 14: E51-6. [ Links ]
15. Lucey S, Lynch CD, Ray NJ, Burke FM, Hannigan A. Effect of preheating on the viscosity and microhardness of a resin composite. J Oral Rehabil. 2010; 37: 278-82. [ Links ]
16. Marchan SM, White D, Smith WA, Raman V, Coldero L, Dhuru V. Effect of reduced exposure times on the microhardness of nanocomposites polymerized by QTH and second-generation LED curing lights. Oper Dent. 2011; 36: 98-103. [ Links ]
17. Dickerson WG, Hastings JH. Indirect composite restorations. Curr Opin Cosmet Dent. 1995; 1: 51-6. [ Links ]
18. Soares CJ, Pizi EC, Fonseca RB, Martins LR.. Mechanical properties of light-cured composites polymerized with several additional post-curing methods. Oper Dent. 2005; 30: 389-94. [ Links ]
19. Poskus LT, Latempa AMA, Chagas MA, Silva EM, Leal MPS, Guimarães JGA. Influence of post-cure treatments on hardness and marginal adaptation of composite resin inlay restorations: an in vitro study. J Appl Oral Sci. 2009; 17: 617-22. [ Links ]
20. da Silva GR, Simamoto-Júnior PC, da Mota AS, Soares CJ. Mechanical properties of light-curing composites polymerized with different laboratory photo-curing units. Dent Mater J. 2007; 26: 217-23. [ Links ]
21. Busato ALS, Arossi GA, Ogliari F, Samuel SMW. The effect of post-cure heating in autoclave, microwave oven and conventional oven on direct composite resin. Rev Odonto Cienc. 2007; 22: 177-80. [ Links ]
22. Trujillo M, Newman SM, Stansbury JW. Use of near-IR to monitor the influence of external heating on dental composite photoactivation. Dent Mater. 2004; 20: 766-77. [ Links ]
23. Santana IL, Gonsalves LM, Lage LM, Lima DM, Pereira AFV, Rodrigues Filho LE. Inlays/Onlays in resin composites for direct use heat treated Part I: description of technique. Rev Bras Pesq Saude. 2010; 12: 76-81. [ Links ] Influence of two methods of additional activation on composite resins surface hardness Braz J Oral Sci. 11(3):396-400.
24. Nomoto R, Asada M, McCabe JF, Hirano S. Light exposure required for optimum conversion of light activated resin systems. Dent Mater. 2006; 22: 1135-42. [ Links ]
25. Bagis YH, Rueggeberg FA. Mass loss in urethane/ TEGDMA and BisGMA/ TEGDMA based resin composites during post cure heating. Dent Mater. 1997; 13: 377-80. [ Links ]
26. Reinhardt JW, Boyer DB, Stephens NH. Effects of secondary curing on indirect posterior composite resins. Oper Dent. 1994; 19: 217-20. [ Links ]
27. Peutzfeldt A. Resin composites in dentistry: the monomer systems. Eur J Oral Sci. 1997; 105: 97-116. [ Links ]
28. Sideridou I, Tserki V, Papanastasiou G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials. 2003; 24: 655-65. [ Links ]
 Correspondence:
Correspondence:
Alessandro Ribeiro Gonçalves
Rua Visconde de Parnaíba 2315, apt 1602,
CEP: 64049-570 - Horto Florestal
Teresina, PI – Brasil
E-mail: argoncalves@yahoo.com
Received for publication: May 12, 2012
Accepted: September 18, 2012













