Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.11 no.3 Piracicaba Jul./Set. 2012
ORIGINAL ARTICLE
Functional activity of neutrophils and systemic inflammatory response of Down's syndrome patients with periodontal disease
Isabelle Rodrigues FreireI; Sandra Maria Herondina Coelho Ávila AguiarII; Sandra Helena Penha de OliveiraIII
IGraduate student in Pediatric Dentistry, School of Dentistry of Araçatuba, UNESP, São Paulo, SP, Brazil
IIProfessor, Department of Pediatric and Community Dentistry, School of Dentistry of Araçatuba, UNESP, São Paulo, SP, Brazil
IIIProfessor, Department of Basic Sciences, School of Dentistry of Araçatuba, UNESP, São Paulo, SP, Brazil
ABSTRACT
Periodontal disease (PD) is characterized as an inflammatory process that compromises the support and protection of the periodontium. Patients with Down's syndrome (DS) are prone to develop PD. Neutrophils (NE) are the first line of defense against infection and their absence sets the stage for disease.
AIM: To compare the activity and function of NE in the peripheral blood from DS patients with and without PD, assisted at the Center for Dental Assistance to Patients with Special Needs affiliated with the School of Dentistry of Araçatuba, Brazil.
METHODS: Purified NE were collected from peripheral blood of 22 DS patients. NE were used to detect the 5-lypoxigenase (5-LO) expression by RT-PCR. Plasma from peripheral blood was collected to measure tumor necrosis factor-a (TNF-α) and interleukin-8 (IL-8) by ELISA and nitrite (NO3) using a Griess assay.
RESULTS: Data analysis demonstrated that DS patients with PD present high levels of TNF-a and IL-8 when compared with DS patients without PD. However, there was no statistically significant difference in the levels of NO3 production between the groups. The levels of the inflammatory mediator 5-LO expression increased in DS patients with PD.
CONCLUSIONS: According with these results, it was concluded that TNF-α and IL-8 are produced by DS patients with PD. Furthermore, DS patients with PD presented high levels of 5-LO expression, suggesting the presence of leukotriene B4 (LTB4) in PD, thus demonstrating that the changes in NE function due to the elevation of inflammatory mediators contribute to PD.
Keywords: Down syndrome, periodontal disease, 5-lypoxigenase, tnf-a.
Introduction
Down Syndrome (DS) is the most common chromosomal aberration resulting from trisomy of the chromosome 21. This trisomy is present in 95% of the phenotypic expression of the DS patients. The remaining cases are due translocation, mosaicism and partial trisomy of the chromosome 21. This phenomenon occurs during spermatogenesis, resulting in three copies of the chromosome 21. Several functional disorders and physical stigmata, such as mental abnormalities, susceptibility to infections, and hypotonic muscle function are associated with this syndrome1-2. DS patients also present a T cell immunodeficiency causing functional defects of polymorphonuclear leukocytes, reduced chemotaxis, diminished phagocytic ability, defective oxidative response and abnormal bactericidal activity3.
DS individuals have an increased prevalence of periodontal disease (PD) compared with otherwise normal, age-matched control groups and other mentally handicapped patients of a similar age4. Signs of alveolar bone loss can be detected in a high percentage of children with DS5-6. The severe periodontal destruction cannot be explained by poor oral hygiene alone7. Meyle and Gonzales8 (2001) described the influence of DS on periodontitis in children and adolescents. It has been suggested that endogenous factors might contribute to the rapid progression of periodontal breakdown such as inappropriate regulation of enzymes, lipid mediators, collagen biosynthesis or T cell immunodeficiency.
PD is highly prevalent and can affect up to 90% of the worldwide population9. Periodontitis results in loss of connective tissue and bone support and is a major cause of tooth loss in adults. The oral cavity is continually exposed to bacteria, their endotoxins and exototoxins, as well as physical stress. This results in a complex microenvironment within the periodontium, consisting of immune surveillance response, cellular damage and repair, and the production of cytokines, chemokines and other inflammatory mediators10. In general, the host response to bacterial stimuli leads to a cascade of inflammatory mediators such as cytokines (TNF-α), chemokines (IL-8) as well as prostaglandins and leukotrienes, which are metabolites from arachidonic acid.
The polymorphonuclear leukocyte-neutrophil is a key cell type and an essential part of the host's inflammatory response. The explanation for the advanced periodontal destruction may include the disturbance in NE chemotaxis. Its protective functions have been studied extensively in relation to the pathogenesis of PD. These functions can be categorized as adherence, chemotaxis, phagocytosis and microbicidal activity3,11-12.
During the inflammatory response, resident cells can release chemotactic mediators that induce neutrophil accumulation and NE mediators' expression such as cytokines, chemokines, lipids-derived mediators and reactive nitrogen species. These molecules are potent stimulants of NE chemotaxis and amplify NE-mediated tissue injury and vascular permeability.
The objective of this study was to compare the activity and function of NE in the systemic inflammatory response of DS patients with PD and the levels of TNF-α, IL-8 and NO3 as well as the expression of 5-LO activity released in peripheral blood from DS patients with and without PD, assisted at a center for dental care to special needs patients.
Material and methods
Participants
Peripheral blood was collected from 22 patients with DS (10 male and 12 females, mean age ± 25.6 ears), being 14 with PD and 8 without, recruited from the patient population of the Center for Dental Assistance to Patients with Special Needs (CAOE-UNESP-FOA), affiliated with the School of Dentistry of Araçatuba, UNESP, Araçatuba, SP, Brazil, in accordance with the protocol approved by the Ethics Committee for human subjects. The patients who participated of this study were previously selected according to an accurate diagnosis of DS by the medical staff, psychologists and therapists who recorded all information regarding systemic health and intellectual level of these patients. The dental team performed the oral examinations and found only a small number of DS patients with periodontal alterations because most of them attended the CAOE-UNESP-FOA and present excellent oral conditions. The peripheral venous blood was collected from patients only after their parents or caregivers receive the necessary information and signed a consent form. The general health of subjects was good and care was taken to ensure that none of them was under anti-inflammatory medication for systemic conditions over the 12 months prior to the study or exhibited any inflammatory process. The patients were clinically diagnosed to evaluate the PD by the dentists of the CAOE-UNESP-FOA.
NE isolation
Peripheral blood NE were isolated by Ficoll-PaqueTM Premium (Invittogen BRL, Life Technologies, Rockville, MD, USA) gradient. In brief, 20 mL of peripheral blood were collected and diluted 1:1 in saline. Subsequently, the diluted whole blood was laid on 15 mL of Ficoll-Paque TM Premium and centrifuged at 450 g for 15 min at 4oC. Afterwards, plasma was collected and contaminating erythrocytes were lysed and NE were suspended in Trizolâ (Invittogen BRL, Life Technologies).
Determination of IL-8 and TNF-α by Enzyme Linked Immuno Sorbent Assay (ELISA)
Plasma from peripheral blood was collected for ELISA in order to measure IL-8 and TNF-α production. Each assay kit (R & D Systems Inc., Minneapolis, MN, USA) contained 96 wells with a microtiter plate coated in monoclonal antibody to TNF-α or IL-8 in the base. Samples were added to the wells and after 2 h, unbound proteins were washed away and an enzyme-linked polyclonal antibody was added to the wells; this antibody acted as a link between the cytokine or chemokine and a dying agent. A color change proportional to the amount of TNF-α or IL-8 was observed. This was quantified by comparing the optical densities of the samples to those of known dilutions using a plate reader at 450 nm. The concentration of each TNF-α and IL-8 was calculated from a standard curve (from 4 to 4000 pg/mL).
Expression of 5-LO by real-time reverse transcription-polymerase chain reaction (RT-PCR)
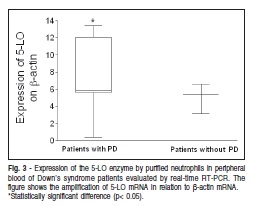
Cells were homogenized with 1 mL of Trizolâ. RNA was extracted with chloroform and centrifuged at 1300g at 4°C for 15 min; before being washed with isopropanol (500 mL) and ethanol (500 mL) following protein precipitation according to the manufacturer's instructions. Complementary DNA (cDNA) was synthesized using 3 mg of RNA through a reverse transcription reaction. Real-time PCR quantitative mRNA analyses were performed in a Rotor Gene 6 using theSYBR-green fluorescence quantification system (Corbett Research, Mortlake, Australia) for quantification of amplicons. The standard PCR conditions were 950C (15 min), and then 40 cycles of 95oC (20 s), 55oC (30 s), 72oC (30 s), and 72oC (1 min), followed by the standard denaturation curve. The sequences of the primers and the predicted amplicon sizes used were as follows: 5-LO sense CCC GGG GCA TGG AGA GCA, antisense GCG GTC GGG CAG CGT, which results in a 561 base pair (bp) amplification product; b-actin sense GGC GAC GAG GCC CAG A, antisense CGA TTT CCC GCT CGG C, which results in a 463 base pair (bp). PCR conditions for each target were thoroughly optimized with regard to primer concentration, absence of primer dimer formation and efficiency of amplification of target genes and housekeeping gene control. SYBR Green PCR Master Mix (Cobertt Research, Mortlake, Australia), 400 nM specific primes and 2.5 ng cDNA were used in each reaction. The positivity of real-time PCR was determined based on negative controls. The relative levels of gene expression were calculated according to the instructions by referring to the b-actin in the sample, using the cycle threshold (Ct) method. Briefly, Ct is the point at which the exponential increase in signal (fluorescence) crosses a somewhat arbitrary signal level (usually 10 times background). The mean Ct values from duplicate measurements were used to calculate the expression of the target gene, with normalization to bactin, and then compared with the target-internal control subjects to calculate the fold increase expression, using the 2- DCt formula. Negative controls without RNA and without reverse transcriptase were also performed. The results show one of three representative experiments.
Determination of production of NO3
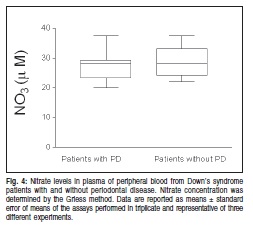
Plasma from peripheral blood was collected for nitrate reduction assay in order to measure NO3 production. Serum samples (40 mL) from DS patients with and without PD were pipetted into the wells of flat-bottomed 96-well microtitration plates, followed by the addition of 40 mL of freshly mixed (500 mL) NADPH (5 mg/mL), 1000 mL KH2PO4, 50 mL nitrate reductase (10 U/500 mL) and 450 mL H2O and left overnight at room temperature. After that, an equal part of Griess reagent was added to the well plates. The plates were shaken for 5 min at room temperature, after which a purple color developed in positive plates. The plates were read in a microplate reader at 540 nm. The concentration of NO2 and NO3 was determined from a standard curve (200 to 0.78 mM) produced using 8 different concentrations of NaNO2 and NaNO3. The results were expressed as mM in triplicate samples.
Chemotactic migration
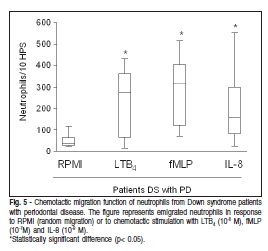
NE were suspended in RPMI supplemented with 0.01% bovine serum albumin at a concentration of 1x106 cells/mL. The cell suspension was placed in the upper compartment of a modified Boyden chamber separated by a 5mm pore-size micropore filter, while the lower compartment was loaded with either the buffer solution or chemoattractant solutions of fMLP (10-7M), LTB4 (10-8M) and IL-8 (10-9 M). The cell migration response was evaluated by enumeration of cells on the distal surface of the filter after one-hour incubation in a 37oC humidified air chamber. Ten representative highpower microscopic fields (x1,000) were counted for each of triplicate filters. The chemotactic migration of NE from DS patients with PD was expressed as an average of the calculated percentage of the chemotactic migration of NE from matched healthy subjects.
Statistical analyses
Student's independent t test (two-tailed) was used to compare the means between the groups as well as the correlation within the group. A general linear ANOVA test was used when testing the correlation coefficients between DS patients with PD and controls. The statistical program PRISMA 3.0 was used. Statistical significance was considered as a two tailed p< 0.05.
Results
The subjects were divided into 2 groups and they were classified by sex, age and intellectual disability. Periodontal status was evaluated by the clinical parameters (Table 1 and 2). In Table 1, it was observed that DS patients with PD are predominantly females, with a mean age of 25 years. However, the patients without PD represented in Table 2, are predominantly males with a mean age of 24 years.
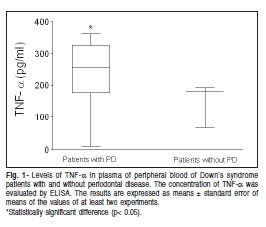
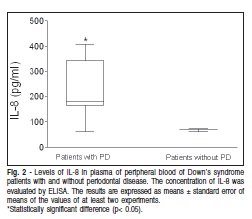
As far as the inflammatory mediators' production in the present study, it was observed that patients with DS and PD have a significantly higher TNF-α production when compared with DS patients without PD (Figure 1). The same pattern of IL-8 production was observed when comparing with DS patients without PD (Figure 2).
The 5-LO expression by purified peripheral NE was evaluated using real time RT-PCR in order to investigate the participation of lipid mediators in the PD of patients with and without DS. It was observed that DS patients with PD showed a higher level of 5-LO expression than DS patientswithout PD (Figure 3). It has been demonstrated11 that leukotrienes are involved in the inflammatory process in periodontitis.
In the next step, nitric oxide (NO) production by nitrate level was assessed and it was found that no significant difference was detected when comparing the NO3 level in DS patients with and without PD (Figure 4).
The chemotaxis assay was used to analyze the functional activity of NE in the DS patients, and it was observed that IL-8, fMLP and LTB4 were chemotactic to NE migration in DS patients in comparison with the control group RPMI (Figure 5).
Discussion
An increase in susceptibility and prevalence of PD in DS patients has been the object of intense study in recent years, as can be observed in current literature12.
Some clinical and laboratory studies have suggested that the genetic abnormalities of patients with DS can modify the systemic response. It includes functional defects of the polymorphonuclear and mononuclear leukocytes, which contribute to the prevalence of PD2.
Oral hygiene is often used as a predictor of patients with PD. Poor oral hygiene directly correlates to the degree of mental retardation as do increased rates of oral diseases among those populations. Limited access to care, limited manual dexterity and lowered efficacy of self homecare, all of these factors contribute to raising levels of gingivitis3. In the present study, we evaluated DS patients of both sexes, ages and degrees of mental abnormalities. Our results support the hypothesis that DS patients with PD present high levels of TNF-α in the peripheral plasma when compared with DS patients without PD. These data strongly suggest that DS patients with periodontitis present an inflammatory disease in due course. These findings are consistent with those of previous studies that reported that high levels of TNF-α were observed in the serum samples of DS patients without periodontal13.
TNF-α is a pluripotent cytokine that plays a central pathogenic role in inflammatory disease. This cytokine is associated with stimulated bone resorption, fibroblast proliferation as well as the production of matrix metalloproteinases and prostaglandin E2 14. Its possible significance in inflammatory PD has been subject of intense research15.
As far as IL-8 is concerned, it was observed that DS patients with PD have a significantly higher level when compared with DS patients without periodontitis. These data are in accordance with literature showing that the mean concentration of IL-8 was higher in the DS group than in the control group14. IL-8 is a chemokine produced by a variety of tissues and blood cells and is a potent inducer of NE chemotaxis and activation16. This cytokine is produced early in inflammation as a result of the interaction of host cells with bacterial stimuli such as lipopolysaccharides (LPS). In inflammatory lesions, the interaction between IL-8 and other inflammatory mediators leads to extravasation and recruitment of NE. An increase in IL-8 expression in periodontal tissue has been previously observed, thus confirming its participation in the periodontitis process17. In the present study, it was found that IL-8 is potentiated in DS patients with PD when compared with literature data.
NO is a molecule that is involved in vascular regulation, homeostasis, bone formation and resorption, neurotransmission and immune function. In recent years, there has been an increasing interest in NO in the pathogenesis of oral and PDs. In the present study, there was no statistically significant difference between the levels of NO in DS patients with PD compared with patients without periodontitis. In the data reported in literature, it is observed that when NO production in the salivary and gingival tissue from patients with periodontitis was measured, it was found to be lower compared with that in healthy samples18. NO production in PD is controversial since the production may be downregulated by arginase19. Overproduction of NO can contribute to tissue damage in periodontal tissues and has been implicated in PD pathogenesis. NO in periodontal tissue is generated mostly via iNOS which catalyze the oxidation of guanidine nitrogen associated with L-arginine. Arginase also uses L-arginine and can down-regulate NO production in saliva and in periodontal tissue17. Since a large amount of NO is toxic to periodontal tissues, arginase may prevent overproduction of NO.
The results of the present study showed that NE from peripheral blood of DS patients with PD significantly express 5-lipoxygenase when compared with patients without periodontitis. The data suggest that the NE from these patients are able to produce leukotriene B4. LTB4 production during the progression of periodontitis has been observed in literature. Gingival tissue from DS subjects presents a high level of this lipid mediator when compared with the control group20.
LTB4 is a proinflammatory mediator that plays an important role in PD. Recently, LTB4 has been implicated as having a role in host defense against microbial infection21. This lipid mediator induces recruitment and activation of NE, monocytes and eosinophils. It also stimulates the production of a number of proinflammatory cytokines and mediators indicating ability to augment and prolong tissue inflammation22. The involvement of LTB4 in PD has been observed in gingival crevicular fluid from subjects with periodontitis23-24.
Defects in NE chemotaxis have also been identified in DS patients25. In addition to the direct involvement of NE in defense against invading pathogens, the NE role in mediating tissue injury plays an important role in the exacerbation of diseases. Under this new paradigm, comparing with our data, the polymorphonuclear leukocyte is not "hypofunctional" or "deficient", but rather "hyperfunctional". The increased activity and the release of toxic products from the cell are responsible, in part, for the tissue destruction in chronic periodontal inflammation26. In our study, we observed that the NE chemotactic activity of IL-8, LTB4 and fMLP is higher when compared with control group. This may suggests that NE is hyper-functional and activated in these DS patients. During the inflammatory cell migration, the chemotactic factors are essential for the beginning and regulation of the increased inflammatory/immune response. In our study, TNFá and IL-8 production as well as 5-LO mRNA expression are more pronounced in DS patients with PD when compared with DS patients without PD in literature data.
NE function may be altered in patients with PD. The improvement in the activity of peripheral blood NE observed in some study is consistent with observed associations between periodontitis and overall health indicators. Intrinsic defects in NE could result in compromised immune conditions, as found in some syndromes. The NE in peripheral blood from patients with PD are defective regarding the production of some mediators of inflammation. The alteration in function refers to an up-regulation of NE, a hyperfunctional activity that may be responsible for the periodontal chronic destruction.
In conclusion the changes in NE function due to the elevation of inflammatory mediators contribute to PD. According to the results of the present research, it may be concluded that:
• the NE chemotactic activity of IL-8, LTB4 and fMLP was higher when compared with the control group;
• the NE is hyper-functional and activated in DS patients;
• the TNF-β and IL-8 production as well as 5-LO mRNA expression are more pronounced in DS patients with PD.
Acknowledgements
This project was supported by grants from the São Paulo State Research Foundation (FAPESP- 2000/08506-0). The authors are indebted to Giuliana Bertozi for her helpful technical assistance and express their gratitude to Dr. Carlos F Santos for his critical reading and suggestions to this manuscript. We also acknowledge Yara Regina Bianchini Ávalos, Alba Valéria Rodrigues Mantovani and Marlene Aparecida Costa for taking care of the patients and collecting peripheral blood samples at the Center for Dental Assistance to Patients with Special Needs (CAOE-UNESP-FOA).
References
1. Forrester MB, Merz RD. Epidemiology of Down syndrome (Trisomy 21), Hawaii, 1986-97. Teratology. 2002; 65: 207-12. [ Links ]
2. Khoshnood B, Wall S, Pryde P, Lee KS. Maternal education modifies the age-related increase in the birth prevalence of Down syndrome. Prenat Diagn. 2004; 24: 79-82. [ Links ]
3. Frydman A, Nowzari H. Down Syndrome - Associated Periodontitis: A critical review of the literature. Compendium. 2012; 33: 356-61. [ Links ]
4. Amano A, Kishima T, Akiyama S, Nakagawa I, Hamada S, Morisaki I. Relationship of periodontopathic bacteria with early-onset periodontitis in Donws's syndrome. J Periodontol. 2001; 72: 368-73. [ Links ]
5. Modéer T, Barr M, Dahllöf G. Periodontal disease in children with Down's syndrome. Scand J Dent Res. 1990; 98: 228-34. [ Links ]
6. Saxén L, Aula S. Periodontal bone loss in patients with Down's syndrome: A follow-up study. J Periodontol. 1982; 53: 158-62. [ Links ]
7. Ulseth JD, Hestnes A, Stavner LJ, Storhaug K. Dental caries and periodontitis in persons with Down's syndrome. Spec Care Dent. 1991; 11: 71-3. [ Links ]
8. Meyle J, Gonzales, J.R. Influence of systemic disease on periodontitis in children. Periodontology 2000. 2001: 92-112. [ Links ]
9. Albandar JM, Rams TE. Periodontol 2000 Global epidemiology of periodontal diseases 29. Copenhagen, Denmark: Munksgaard .Blackwells; 2002. [ Links ]
10. Khocht A, Yaskell T, Janal M, Turner BF, Rams TE, Haffajee AD et al. Subgingival microbiota in adult Down syndrome periodontitis. J Periodontal Res. 2012; 47: 500-7. [ Links ]
11. Carneiro MV, Bezerra AC, Guimarães MD, Muniz-Junqueira MI. Effects of periodontal therapy on phagocytic of peripheral blood neutrophilsevidence for an extrinsic celular defect. Oral Health Prev Dent. 2012; 10: 195-203. [ Links ]
12. Scott DA, Krauss J. Neutrophils in neutrophils inflammation. Front Oral Biol. 2012; 15: 56-83. [ Links ]
13. Komatsu T, Kubota M, Sakai N. Enhancement of matrix metalloproteinase (MMP)-2 activity in gingival tissue a culture fibroblasts from Down's syndrome patients. Oral Dis. 2001; 7: 47-55. [ Links ]
14. Górska DT, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, Madaliñski K. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol. 2003; 30: 1046-52. [ Links ]
15. Yucel-Lindberg T, Twetman S, Skold-Larsson K, Modeer T. Effect of an antibacterial dental varnish on the levels of prostanoids leukotriene B4, and interlekin-1 beta in gingival crevicular fluid. Acta Odontol Scand. 1999; 57: 23-7. [ Links ]
16. Galbraith GMP, Hagan C, Steed RB, Sanders JJ, Javed T. Cytokine Production by oral and peripheral blood neutrophils in adult periodontitis. J. Periodontol. 1997; 68: 832-8. [ Links ]
17. Nelson PG, Kuddo T, Song YE, Dambrosia JM, Kohler S, Satyanarayana G, et al. Selected neutrophils, neuropeptides, and cytokines: developmental trajectory and concentrations in neonatal blood of children with autism or Down syndrome. Int J Dev Neurosci. 2006; 24: 73-80. [ Links ]
18. Shimauchi H, Takayama S, Narikawa-Kiji M, Shimabukuru Y, Okada H. Production of Interleukin-8 and Nitric Oxide in Human Periapical Lesions. J Endod. 2001; 27: 749-52. [ Links ]
19. Garlet GT , Martins Jr. W, Ferreira BR , Milanezi CM, Silva JS. Patterns of Chemokines and Chemokine receptors expression in different forms of human periodontal disease. J. Periodont Res. 2003; 38: 210-17. [ Links ]
20. Ugar-Çankal D,Ozmeric N. A multifaceted molecule, nitric oxide in oral and periodontal disease. Clin Chim Acta. 2006; 366: 90-100. [ Links ]
21. Güllü C, Ozmeric N, Tokman B, Elgün S, Balos K. Effectiveness of scaling and root planning versus modified Wildman flap on nitric oxide synthase and arginase activity in patients with chronic periodontitis. J Periodont Res. 2005; 40: 168-75. [ Links ]
22. Tsilingaridis G, Yucel-Lindberg T, Modéer T. Enhanced levels of prostaglandin E2, Leukotriene B4, and matrix metalloproteinase-9 in gingival fluid from patients with Down syndrome. Acta Odontol Scand. 2003; 61: 154-8. [ Links ]
23. Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter R, Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol. 1996; 157: 5221-4. [ Links ]
24. Crooks WS, Stockley AR. Leukotriene B4. Int J Biochem Cell Biol. 1998; 30: 173-8. [ Links ]
25. Emingil G, Cinarcik S, Baylas H, Coker I, Hüseyinov A. Levels of leukotriene B4 in gingival crevicular fluid and gingival tissue in specific periodontal disease. J Periodontol. 2001; 72: 1025-30. [ Links ]
26. Bäck M, Airila-Mansson S, Jogestrand T, Söder B, Söder PO. Increased leukotriene concentrations in gingival crevicular fluid from subjects with periodontal disease and atherosclerosis. Atherosclerosis. 2007; 193: 389-94. [ Links ]
 Correspondence:
Correspondence:
Sandra Maria Herondina Ávila Coelho Aguiar
Pediatric Dentistry and Social Department
School of Dentistry of Araçatuba, UNESP
Rua José Bonifácio, 1193 - Vila Mendonça -
CEP: 16015-050, Araçatuba-SP, Brasil
E-mail: saguiar@foa.unesp.br













