Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.11 no.4 Piracicaba Out./Dez. 2012
ORIGINAL ARTICLE
Morse taper implants at different bone levels: a finite element analysis of stress distribution
Marcelo Bighetti ToniolloI; Ana Paula MacedoII; Daniel PalharesIII ; Paulo Linares CalefiIV; Danilo Balero SorginiV; Maria da Gloria Chiarello de MattosVI
ISpecialist in Prosthodontics; Master of Science and PhD student of Oral Rehabilitation at the Dental School of Ribeirão Preto – University of São Paulo, Department of Dental Materials and Prosthodontics, Ribeirão Preto, SP, Brazil
IIMaster of Science and PhD student at the School of Medicine of Ribeirão Preto – University of São Paulo; Technical Responsible of the Metrology Laboratory of Dental School of Ribeirão Preto – University of São Paulo, Department of Dental Materials and Prosthodontics, Ribeirão Preto, SP, Brazil
IIISpecialist in Prosthodontics; Master of Science student of Health Sciences – Implantology – Unifeb, Barretos, SP, Brazil
IVSpecialist in Prosthodontics; Master of Science student of Oral Rehabilitation at the Dental School of Ribeirão Preto – University of São Paulo, Department of Dental Materials and Prosthodontics, Ribeirão Preto, SP, Brazil
VSpecialist in Prosthodontics; Master of Science and PhD student of Oral Rehabilitation at the Dental School of Ribeirão Preto – University of São Paulo, Department of Dental Materials and Prosthodontics, Ribeirão Preto, SP, Brazil
VIFull professor at the Dental School of Ribeirão Preto – University of São Paulo, Department of Dental Materials and Prosthodontics, Ribeirão Preto, SP, Brazil
ABSTRACT
AIM: To explore the biomechanical effects of the different implantation bone levels of Morse taper implants, employing a finite element analysis (FEA).
METHODS: Dental implants (TitamaxCM) with 4x13 mm and 4x11 mm, and their respective abutments with 3.5 mm height, simulating a screwed premolar metal-ceramic crown, had their design performed using the software AnsysWorkbench10.0. They were positioned in bone blocks, covered by 2.5 mm thickness of mucosa. The cortical bone was designed with 1.5 mm thickness and the trabecular bone completed the bone block. Four groups were formed: group 11CBL (11 mm implant length on cortical bone level), group 11TBL (11 mm implant length on trabecular bone level), group 13CBL (13mm implant length on cortical bone level) and group 13TBL (13 mm implant length on trabecular bone level). Oblique 200 N loads were applied. Von Mises equivalent stresses in cortical and trabecular bones were evaluated with the same design program.
RESULTS: The results were shown qualitatively and quantitatively by standard scales for each type of bone. By the results obtained, it can be suggested that positioning the implant completely in trabecular bone brings harm with respect to the generated stresses. Its implantation in the cortical bone has advantages with respect to better anchoring and locking, reflecting a better dissipation of the stresses along the implant/bone interfaces. In addition, the search for anchoring the implant in its apical region in cortical bone is of great value to improve stabilization and consequently better stress distribution.
CONCLUSIONS: The implant position slightly below the bone in relation to the bone crest brings advantages as the best long-term predictability with respect to the expected neck bone loss.
Keywords: biomechanics, bone, dental implants, finite element analysis.
Introduction
The use of dental implants in contemporary dentistry has become a reality, bringing many solutions, but also problems related to various factors (both clinical and biomechanical)1-2. Because the proper functioning of implants is based onosseointegration, the relationship between biomechanical implants and the surrounding hard tissue is of great importance, as well as its spatial positioning and implantation insertion depth.
The quality and type of bone involved in implantology have been extensively studied3-7, and is clear that there is importance and relevance of these factors in effective and appropriate force dissipation generating or not a favorable prognosis to the implants. The basic biomechanical difference between cortical and trabecular bones is linked to their different modulus of elasticity, as well as their different types, and this is where studies involving finite elements differ in their analyzes5,7-10. The higher the modulus of elasticity in simulated analysis, the greater the simulated bone density (type I to type IV).
De Almeida et al.7 obtained results with the highest maximum principal stress in bones of type III and IV. Three unilateral posterior loads of 150 N were used (perpendicular to the prefabricated bar; 30 degrees in a buccolingual direction and 30 degrees in a linguobuccal direction). They also concluded that the bone type should not exclusively be the only determining factor in stress distribution. These authors also affirmed that there are various other factors that influence the pattern of stress distribution, such as implant design, length and diameter, applied forces, implant insertion depth and type of internal connection.
The Morse taper implant has been highlighted on several positive features, such as its ability to decrease bacterial contamination between implant and prosthesis, more aesthetic predictable and biological quality of the peri-implant tissue, in addition to reducing the risk of loosening the prosthetic screw11-12. Quaresma et al.13 also showed that the Morse taper connection dissipates less stress to the implant surrounding bone than the internal hexagonal connection. Moreover, some manufacturers' recommendation is that the best positioning of Morse taper implants has to be slightly below the bone in relation to the bone crest; however if it is placed too deep, it can bring too many differences regarding the stresses distribution.
Evidence found by Akça and Cehreli14 revealed that the gradual loss of marginal bone around the implant led to considerable increase of stress in trabecular bone in contact with the cervical region of the implant. This simulated effect of bone resorption would be equivalent to the deeper implantation of the implant, since the loss of cortical bone would result in only trabecular bone support of the implant, which has different characteristics from the first one. Similar findings were found in Okumura et al.15 study.
The aim of this study was to explore the biomechanical effects in the peri-implant bone of the different implantation bone levels of Morse taper implants.
Material and methods
The implants used in this study (Profile Projector - Nikon Model 6C and Stereomicroscope - Leica Model S8AP0) were measured so that it could have higher degree of fidelity. The implants and respective abutment used are shown in Table 1 and Figure 1. It was standardized only 4 mm for implant diameter in order not to create another variable and only evaluate the correlation between implant length and different implantation bone levels. The implant prostheses were designed to be screwed premolar metal-ceramic crowns. Three-dimensional finite element graphic models reproduction of all prosthetic elements and implants required for this study, as well as a bone block in which the implants were inserted, were performed using the program AnsysWorkbench10.0 (Swanson, Analysis Systems, Inc., Houston, TX, USA).


The implants were positioned in bone blocks, "covered" of 2.5 mm thickness of mucosa. The cortical bone was designed with 1.5 mm thickness and the trabecular bone completed the bone block, both configuring a 1,742 mm3 total volume. Four groups were formed: group 11CBL (11 mm implant length on cortical bone level), group 11TBL (11 mm implant length on trabecular bone level), group 13CBL (13 mm implant length on cortical bone level) and group 13TBL (13 mm implant length on trabecular bone level) (Figure 2).
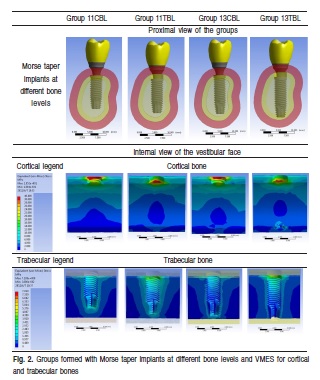
The results were analyzed by a color scale, where each tone corresponds to an amount of stress generated in the structures, and how they were distributed over the analyzed structures (implants, abutments, bone, or any other object ofanalysis) in the three directions of space (X, Y and Z).
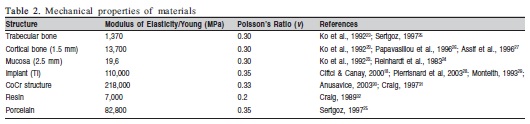
Oblique loads (approximately 45o) in the linguobuccal direction with 200 N of intensity were applied and von Mises equivalent stresses (VMES) on cortical and trabecular bones were evaluated. All the specific properties of each structure involved in the simulations (Modulus of Elasticity/Young and Poisson's Ratio) are presented in Table 2. Standard scales for each type of bone were constructed to analyze qualitatively and quantitatively. It was considered the internal face of the buccal side for stress analysis because of their greater relevance considering the direction of oblique forces.
In the absence of information about the precise organic properties of the cortical and trabecular bones and mucosa, they were assumed to be homogeneous, isotropic, and linearly elastic as were the other materials used in the analysis.
Results
The VMES were evaluated, which represents the mean of the stresses in all directions, in different groups. Quantitative comparisons were made between the different groups to determine the generated stresses in cortical and trabecular bones (Figure 2).
The VMES values ranged from 0 to 40 MPa in cortical bone, and from 0 to 7 MPa in trabecular bone, approximately. In general, the absolute values of stress on cortical bone were the same, but with greater width in the groups with cortical bone level implant (CBL). For the trabecular bone stress, it was higher in the groups with trabecular bone level implant (TBL) and reaching higher absolute values than CBL.
Discussion
The objective of this study, using three-dimensional finite element analysis (FEA), was not to replicate exact in vivo stresses, but rather to illustrate a possible difference of the stress distribution on different bone levels of Morse taper implants. FEA allows a better understanding of implants' biomechanical aspects and how such stress occurs on the surrounding bone. Long-term clinical research is required to determine the influence of observed stress levels on implants and surrounding bone16.
Bone quality has been considered the most critical factor for implant success at both surgical and functional stages, and it is therefore suggested that occlusal overload in poor quality bone can be a clinical concern for implant longevity3. In studies n human patients, higher failures of implants were observed in bone with poor quality8-9. Tada et al.5 also confirms the importance of bone quality and its pre-surgical diagnosis for implant long-term prognosis. The results of their study suggest that trabecular bone with higher bone density might ensure a better biomechanical environment for implants.
Rubo and Souza17 concluded in FEA that stresses tended to be concentrated at the cortical bone around the cervical region of the implant closest to the load, whereas stresses in trabecular bone were considered low.
Contrary to the findings of Baggi et al.18 and the results in this study, Chou et al.19 concluded that, evaluating the biomechanical response of the jaw bone with wide-diameter and short implants versus narrow-diameter and long implants, using FEA, there was more even and higher strain distribution in the peri-implant bone at the wide-diameter and short implant as compared with the narrow-diameter and long implant, apart from the fact that stress levels in peri-implant bone were reduced as the insertion depth of the implant was increased. These findings may be due to the comparative association just of the wide-diameter and short implants to the narrow-diameter and long implants. The short length of the implants may have been superimposed on the beneficial effect of large diameter, also interfering with the stress in relation to the insertion depth.
Undue tensions, originated from a possible occlusion maladjusted, poorly positioned implant or even due to the presence of poor quality bone, can lead to injury or bone resorption20. According to Isidor6, occlusal forces affect an oral implant and the surrounding bone, and according to the bone physiology theories, bones carrying mechanical loads adapt their strength to the load applied on it by bone modeling/remodeling. The phenomenon of bone resorption in the form of a saucer around the cervical region of implants called saucerization may arise from both exacerbated tensions in the region or even local biological factors, such as bone loss is observed also in non-loaded implants21.
Another possible cause of this bone loss is related to the low stresses acting on the peri-implant bone. An equivalent stress of 1.6 MPa has been deemed sufficient to avoid crestal bone loss from disuse atrophy in the mandibular canine-premolar region22.
Akça and Cehreli14 simulated by FEA the gradual loss of peri-implant bone. The authors concluded that this loss is highly prejudicial to the biomechanical system. The presence of cortical bone contacting a load-carrying implant, even in a bone defect, improves the biomechanical performance of implants in comparison with only trabecular bone support as a sequel of progressive marginal bone loss. These findings are totally in favor of the results present in this study.
Pierrisnard et al.23 specifically studied the bicortical anchorage effect on the transferred stress to implant components, the implant proper, and the surrounding bone. Such as in this study, the authors showed that the stress concentrated to the cortical bone, at the cervical area, and affirmed that the use of long implants (more than 10 mm) is a positive factor in osseointegration. However, this does not always result in better stress distribution to the implant components and bone, as if the cervical portion of the cortical anchoring of the implant is good, the influence of implant length becomes less important.
In this study, stresses were higher in cortical bone, but this should not represent a risk factor because it was expected due to its higher modulus of elasticity. This finding was also found by Baggi et al.18, and that implant biomechanical behavior greatly improves efficiently if bone is preserved at the crest. The literature reports various values of the elastic and plastic boundary deformation, which may be mentioned an average of 140 MPa for cortical bone and 10 MPa for trabecular bone24-25. Trabecular bone stresses were higher when the implants were at this level (TBL), and better distributed for greater implant length and anchored with its apex in the cortical bone.
Qian et al.26 investigated the interactions between diameter, insertion depth, and load angle applied on the implant by three-dimensional finite element analysis. The authors concluded that a narrow-diameter implant, when inserted into jawbone with a shallow insertion depth and loaded with an oblique loading angle, is most unfavorable for stress distribution in both bone and implant. This result may have been found because with greater depth of implant installation the lever arm portion of the prosthesis has less effect. However, it has also to be considered that the implant in the cortical bone tends to increase its stability and locking, as reported by Pierrisnard et al.23.
Okumura et al.15 performed a FEA to investigate the effect of maxillary cortical bone thickness, implant design and diameter on stress around implants and the von Mises stresses were calculated. Regardless of load direction, implant design and diameter, cortical and trabecular bone stresses increased with the decrease of crestal cortical bone thickness. In the absence of crestal cortical bone, trabecular bone stresses were highest and, under axial load, were transferred to the sinus floor. From a biomechanical viewpoint, to improve implant success odds in the posterior maxilla, rather than implant selection, careful preoperative evaluation of the cortical bone at the planned implant site is recommended. If this cortical bone is too thin or even lacking, implanttreatmentshould be carried on with caution by progressive loading in the range of functional loads.
Thus, just as observed by Akça and Cehreli14, Okumura et al.15 and Pierrisnard et al.23, the loss of cortical bone at the cervical region of the implant can cause biomechanical harm at the implant/bone interface.
Within the limitations of this in vitro study, the following conclusions can be drawn:
• According to the literature, the implant position slightly below the bone in relation to the bone crest brings advantages already known as the best long-term predictability with respect to the expected bone loss around its cervical region, but also the best behavior of soft periimplant tissue;
• However, by the results obtained, it can be suggested that the positioning of the implant completely in trabecular bone brings harm with respect to the generated stresses. Its implantation in the cortical bone has advantages with respect to better anchoring and locking, reflecting the better dissipation of the stresses along the implant/bone interfaces.
• In addition, the search for anchoring the implant in its apical region in cortical bone is of great value to improve stabilization and consequently better stress distribution.
References
1. Goodacre CJ, Bernal G, Rungcharassaeng K, Kan JYK. Clinical complications with implants and implant prostheses. J Prosthet Dent. 2003; 90: 121-32. [ Links ]
2. Davarpanah M, Martinez H, Tecucianu JF, Celletti R, Lazzara R. Smalldiameter implants: indications and contraindications. J Esthet Dent. 2000; 12: 186-94. [ Links ]
3. Misch CE. Density of bone: effect on treatment plans, surgical approach, healing, and progressive loading. J Oral Implant. 1990; 6: 22-31. [ Links ]
4. Akagawa Y, Sato Y, Teixeira ER, Shindoi N, Wadamoto M. A mimic osseointegrated implant model for three-dimensional finite element analysis. J Oral Rehabil. 2003; 30: 41-5. [ Links ]
5. Tada S, Stegaroiu R, Kitamura E, Miyakawa O, Kusakari H. Influence of implant design and bone quality on stress/strain distribution in bone around implants: a 3-dimensional finite element analysis. Int J Oral Maxillof Implants. 2003; 18: 357-68. [ Links ]
6. Isidor F. Influence of forces on peri-implant bone. Clin Oral Impl Res. 2006; 17: 8-18. [ Links ]
7. De Almeida EO, Rocha EP, Freitas ACJR, Martin MJR. Finite element stress analysis of edentulous mandibles with different bone types supporting multipleimplant superstructures. Int J Oral Maxillofac Implants. 2010; 25: 1108-14. [ Links ]
8. Jaffin RA, Berman CL. The excessive loss of Branemark fixtures in type IV bone: a 5-year analysis. J Periodontol. 1991; 62: 2-4. [ Links ]
9. Becktor JP, Eckert SE, Isaksson S, Keller EE. The influence of mandibular dentition on implant failures in bone-grafted edentulous maxillae. Int J Oral Maxillofac Implants. 2002; 17: 69-77. [ Links ]
10. Guan H, Staden R, Loo YC, Johnson N, Ivanovski S, Meredith N. Incluence of bone and dental implant parameters on stress distribution in the mandible: a finite element study. Int J Oral Maxillofac Implants. 2009; 24: 866-76. [ Links ]
11. Sutter F, Weber H, Sorensen J. The new restorative concept of the ITI dental implant system: design and engineering. Int J Periodontics Restorative Dent. 1993; 13: 409-31. [ Links ]
12. Binon PP, Sutter F, Beaty K, Brusnki J, Gulbransen H, Weiner R. The role of screws in implant systems. Int J Oral Maxillofac Implants. 1994; 9(Suppl): 48-63. [ Links ]
13. Quaresma SET, Cury PR, Sendyk WR, Sendyk C. A finite element analysis of two different dental implants: stress distribution in the prosthesis, abutment, implant, and supporting bone. J Oral Implantol. 2008; 34: 1-6. [ Links ]
14. Akça K, Cehreli MC. Biomechanical consequences of progressive marginal bone loss around oral implants: a finite element stress analysis. Med Bio Eng Comput. 2006; 44: 527-35. [ Links ]
15. Okumura N, Stegaroiu R, Kitamura E, Kurokawa K, Nomura S. Influence of maxillary cortical bone thickness, implant design and implant diameter on stress around implants: A three-dimensional finite element analysis. J Prosthodont Res. 2010; 54: 133-42. [ Links ]
16. Tawil P, Tawil G. Short implants in deficient posterior jaws: current knowledge. Implant Dent. 2009; 46: 9-16. [ Links ]
17. Rubo JH, Souza EAC. Finite element analysis of stress in bone adjacent to dental implants. J Oral Implantol. 2008; 34: 248-255. [ Links ]
18. Baggi L, Cappelloni I, Girolamo MD, Maceri F, Vairo G. The influence of implant diameter and length on stress distribution of osseointegrated implants related to crestal bone geometry: A three-dimensional finite element analysis. J Prosthet Dent. 2008; 100: 422-31. [ Links ]
19. Chou HY, Muftu S, Bozkava D. Combined effects of implant insertion depth and alveolar bone quality on periimplant bone strain induced by a wide-diamenter, short implant and a narrow-diameter, long implant. J Prosthet Dent. 2010; 104: 293-300. [ Links ]
20. Erkmen E, Meriç G, Kurt A, Tunç Y, Eser A. Biomechanical comparison of implant retained fixed partial dentures with fiber reinforced composite versus conventional metal frameworks: A 3D FEA study. J Mech Behav Biomed Mater. 2010; 4: 107-16. [ Links ]
21. Consolaro A, Carvalho RS, Francischone Jr CE, Consolaro MFMO, Francischone CE. Dental implants saucerization and orthodontic clinical cases. Dent Press J Orthod. 2010; 15: 19-30. [ Links ]
22. Geng J, Tan KBC, Liu G. Application of finite element analysis in implant dentistry: A review of the literature. J Prosthet Dent. 2001; 85: 585-98. [ Links ]
23. Pierrisnard L, Renouard F, Renault P, Barquins M. Influence of implant length and bicortical anchorage on implant stress distribution. Clin Implant Dent Relat Res. 2003; 5: 254-62. [ Links ]
24. Martens M, Audekercke RV, Delport P, Meester PD, Mulier JC. The mechanical characteristics of cancellous bone at the upper femoral region. J Biomech. 1983; 16: 971-83. [ Links ]
25. Jensen NC, Madsen LP, Linde F. Topographical distribution of trabecular bone strength in the human os calcanei. J Biomech. 1991; 24: 49-55. [ Links ]
26. Qian L, Todo M, Matsushita Y, Koyano K. Effects of implant diameter, insertion depth, and loading angle on stress/strain fields in implant/jawbone systems: finite element analysis. Int J Oral Maxillofac Implants. 2009; 24: 877-86. [ Links ]
 Correspondence:
Correspondence:
Marcelo Bighetti Toniollo
Departamento de Materiais Dentários e Prótese,
Universidade de São Paulo
Av. José Adriano Arrobas Martins, 130,
Nova Aparecida, CEP: 14883-298
Jaboticabal, SP, Brasil
E-mail: martoniollo@yahoo.com.br
Received for publication: June 17, 2012
Accepted: September 18, 2012













