Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.12 no.1 Piracicaba Jan./Mar. 2013
ORIGINAL ARTICLE
Morphological aspects of dentin-pulp complex development in the offspring of rats treated with fluoxetine during pregnancy
Isabela Maria de Albuquerque SantiagoI; Luciana Silva RegueiraI; Priscylla Gonçalves CorreiaI; Robério José Barbosa de AlcântaraII; Joaquim Evêncio NetoIII; Liriane Baratella-EvêncioIV
IDepartment of Dentistry, Federal University of Pernambuco, Recife, PE, Brazil
IIDental Surgeon, Recife, PE, Brazil
IIIDepartment of Morphology and Animal Physiology, Federal Rural University of Pernambuco, Recife, PE, Brazil
IVDepartment of Histology and Embryology, Federal University of Pernambuco, Recife, PE, Brazil
ABSTRACT
AIM: To evaluate the morphological aspects of coronal dentinogenesis in the first molars of 1- and 5-day-old rats whose mothers were treated with fluoxetine hydrochloride during pregnancy.
METHODS: Twelve pregnant Wistar rats were divided randomly into three groups: group C (control), group FL (fluoxetine administered at 10 mg/kg bodyweight), and group FX (fluoxetine administered at 20 mg/kg bodyweight). Saline (0.9%) solution or fluoxetine hydrochloride was administered subcutaneously for the first 21 days of pregnancy. Subsequently, the offspring of these animals was subdivided into subgroups according to age of tooth germ development to be studied: 1 and 5 days of life. C1 and C5 (control group 1 and 5 days of age); FL1 and FL5 (groups treated with 10 mg/kg fluoxetine at 1 and 5 days of age); FX5 and FX1 (groups treated with 20 mg/ kg fluoxetine at 1 and 5 days of age).
RESULTS: No structural changes in the dentin-pulp complex of rats whose mothers were treated with fluoxetine hydrochloride were observed at either dose.
CONCLUSIONS: Fluoxetine, at the doses administered during pregnancy in this study, did not alter the morphological development of the coronal dentin-pulp complex in their offspring.
Keywords: fluoxetine, serotonin, dental germ, dentin-pulp complex.
Introduction
Clinical depression is a recognized public health problem, interfering decisively in the personal, professional, social and economic standing of patients. In recent years, a greater number of women during pregnancy have been diagnosed with depression. The pharmacological treatment of this disease has reduced morbidity and improved the clinical outcomes of thousands of patients with depression globally1-2.
Several drugs cross the placental barrier and some have teratogenic effects on the fetus, which may cause dysfunction and disorders during embryonic development, particularly during the first three months of intrauterine life2.
In 1987, the US Food and Drug Administration approved the first antidepressant (fluoxetine) in a group of selective serotonin reuptake inhibitors (SSRIs)3. Fluoxetine hydrochloride is the most widely prescribed antidepressant in the world, which acts by inhibiting serotonin (5-HT) reuptake and enhancing serotonergic neurotransmission4-5.
The 5-HT neurotransmitter regulates important pathways of mammalian metabolism and is synthesized from the phenylalanine, tyrosine and tryptophan amino acids5.
In the human brain, the first 5-HT-releasing neurons are present from the fifth week and their numbers increase markedly by the tenth week of gestation6. 5-HT interacts with its receptors, which alters cell metabolism and influences several stages of organogenesis7. Serotonergic neurotransmission modulates cell proliferation in several tissues, but is involved mainly in the morphogenesis of the craniofacial region8.
The metabolism of mineralized tissues can be influenced by the central nervous system (CNS)9. Neuroendocrine mechanisms, particularly those related to 5-HT, are associated with the differentiation and activation of osteoblasts and osteoclasts. Reports have demonstrated that fluoxetine hydrochloride can induce bone resorption in rats by blocking 5-HT reuptake10.
5-HT also has a stimulatory role in tooth germ development by inducing the formation of enamel and dental papilla to form the bell and crown stages. These phenomena are cyto- and histo-differentiation steps that occur during odontogenesis and give rise to teeth11-12.
Extensive renovation of the epithelium, cell proliferation, apoptosis and changes in the shape and positioning of cell groups are determined by morphogenetic gradients that play critical roles during the morphogenesis of teeth13.
The tooth germ develops in five stages: the bud, cap, bell, crown and root. After bell formation is complete, the tooth germ has the required structure to form the tooth and its supportive and protective tissues14. The beginning of the crown phase is characterized by odontoblast maturation and deposition of dentin, followed by enamel secretion by ameloblasts to form the mineralized tissues of the future crown15. Therefore, it is possible that chronic exposure to therapeutic doses of SSRIs alters the development of the serotonergic system. However, such evidence is yet unclear and more studies are required.
The objective of this study was to evaluate the morphological aspects of coronal dentinogenesis in the first molars of 1- and 5-day-old rats whose mothers were treated with fluoxetine hydrochloride during pregnancy.
Material and methods
This study was conducted at the Animal facility of the Center for Experimental Surgery at the Department of Histology and Embryology and at the Laboratory of Histotechnical Graduate Programs in Pathology, Federal University of Pernambuco. The experiment protocol was approved by the Committee for Ethics in Animal Research of the Center for Biological Sciences, Federal University of Pernambuco, Recife, Brazil, number 23076.006899/2008 - 51.
Twelve female albino Wistar rats received a standard animal care facility diet and water ad libitum. They were kept in a room maintained at 23 ± 2°C and with a 12:12 h light/dark cycle. After mating and pregnancy confirmation by vaginal smear, the rats were divided into three groups, including four in a control group (C group), four in a group treated with fluoxetine at 10 mg/kg bodyweight (FL group) and four in a group treated with fluoxetine at 20 mg/kg bodyweight (FX group). Each group was subdivided into two subgroups according to the age of the offspring (1 or 5 days of age), totaling six subgroups, according to the Figure 1.
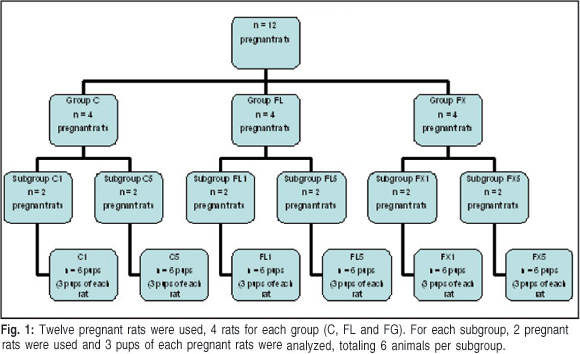
Two pregnant rats were used for each subgroup and each litter was used for three pups of both genders. Six animals in each subgroup were obtained from pregnant rats treated according to the study groups from which they belonged for the first 21 days of pregnancy. In group C, mothers received 0.9% saline at 10 mL/g injected subcutaneously daily between 7 and 8 am. Mothers in the FL and FX groups were treated daily with fluoxetine injected subcutaneously using the same protocol established for the control group.
Animals at 1 day of age were cryo-anesthetized and those at 5 days of age were anesthetized by subcutaneous injection of ketamine (25 mg/kg) and xylazine (10 mg/kg). The animals were decapitated, their jaws removed and the maxillary first molar with the left and right tooth germs were sectioned tangentially to the mesial of the first molar. Specimens were fixed in 10% buffered formalin for 24 h at room temperature. Then, the 5-day-old specimens were decalcified in an aqueous 10% nitric acid solution followed by conventional histology and embedding in paraffin. Serial 4-µιη thick sections were prepared, stained with hematoxylin-eosin, mounted in Entellan® mounting medium (Merck KGaA, Darmstadt, Germany), were and observed and photographed under an Eclypse 51® optical microscope (Nikon, Tokyo, Japan).
The crown of each tooth germ was divided into cusp, middle and cervical thirds. The histological characteristics of the dentin, pre-dentin and odontoblastic layers were observed and described in these regions.
The Eclypse 51 ® optical microscope (Nikon, Tokyo, Japan). with a miniature camera connected to a computer containing an image capture board (ATI) and the IMAGE J® software (National Institute of Mental Health, Bethesda, MD, USA) was used for the histometric analysis. The crowns were analyzed, and each tissue was assigned 10 fields in which the thicknesses of the pre-dentin and dentin as well as the height of odontoblasts were measured. The means and standard deviations of these data were calculated for statistical analysis. The statistical analysis was performed using a oneway ANOVA and Tukey's test. The level of significance was set at p<0.05.
Results
One-day-old animals
It was observed that the tooth germ was in the initial of crown stage. Initial deposition of a very thin dentin matrix with light pink coloration was noted in the third cuspidate. The palisade odontoblastic layer with highly prismatic aspects and a nucleus polarized toward the dental papilla was observed beneath the dentin matrix. The odontoblast cell layer resembled pseudo-stratified epithelium in the region of the cusp curvature due to the proximity of the cells. The preameloblast cell layer, which has highly prismatic cells, was observed above the dentin matrix, but the synthesis of enamel matrix had not yet begun (Figure 2).
Dentin matrix was observed in the middle third down the slope of the crown, as thick as the third cuspidate, which tapered toward the cervical third. The odontoblasts underlying this layer were less prismatic, but the nuclei were biased toward the dental papilla, indicating cytodifferentiation activity. In the same way, the preameloblasts were prismatic, but less so than those in the anterior third (Figure 2).
No deposition of dentin matrix was observed in the cervical third, and dental papilla cells were more condensed and in the cellular differentiation phase. Such cells had a star appearance, were basophilic and had rounded nuclei. Odontoblasts and preameloblasts were observed in this region, and the inner epithelium of the enamel organ cells had a low prismatic or cuboid aspect with a central nucleus (Figure 2).
No structural differences in the tooth germ were observed among the groups of animals of this age. No significant changes in the thickness of the deposited dentin matrix were observed when compared to the C1, FL1, and FX1 subgroups. No significant changes in the length of the odontoblast cells in the region of the future cusp on the middle third of the crown were observed in any of the subgroups (Figure 3).
Five-day-old animals
It was observed that the tooth germ was at an advanced crown stage. No structural differences in tooth germ were observed in the animals of this age in the three study groups.
The dentin layer was thicker in the third cuspidate than in the younger group, with a dark pink color, and the pre-dentin layer was light pink. The enamel matrix was stained purple-blue above the dentin layer. Highly prismatic ameloblasts above the enamel matrix were observed. Below the pre-dentin layer, odontoblasts were observed arranged in a palisade pattern with high prismatic aspects and polarized nuclei toward the dental papilla. The odontoblastic cell layer resembled a pseudo-stratified epithelium in the cusp curvature region, due to proximity and rearrangement of the cells (Figure 4).
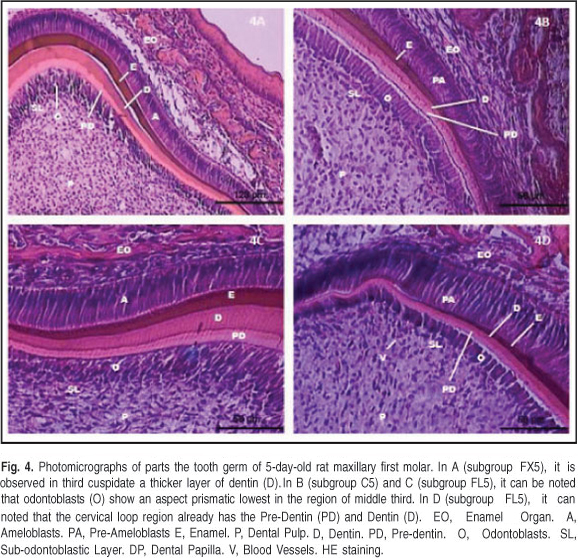
In the middle third, down the slope of the crown, were observed the predentin and dentin layers, which were thinner than the cuspidate, tapered toward the cervical third. The odontoblasts underlying the predentin layer were prismatic with polarized nuclei toward the dental papilla, but lower than those in the third cusp. Above the dentin layer was the enamel matrix, thinner in the anterior third. Prismatic ameloblasts above the enamel matrix were observed, but lower than those in the anterior third (Figure 4).
Dentin and pre-dentin layers in the cervical third were found, but they were thinner than those in the cusp and middle third. Above the dentin layers, preameloblasts with prismatic features were observed but no enamel matrix in this region. Below the predentin layer, the odontoblast layer with a low prismatic aspect and nuclei toward the dental papilla was found, indicating synthesis activity (Figure 4).
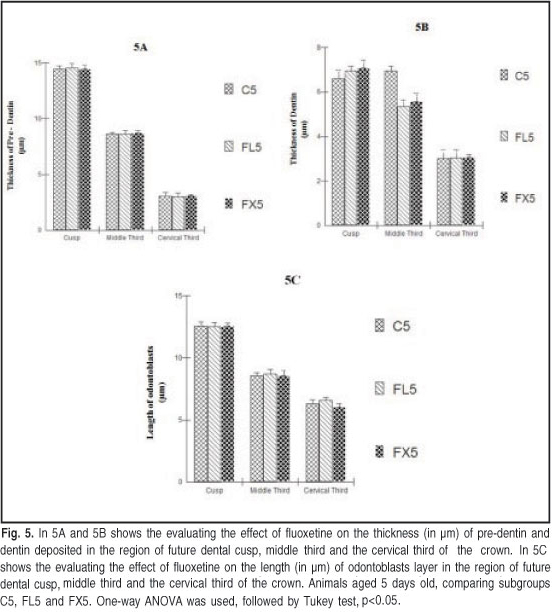
No structural differences in tooth germ were observed in the animals of this age in the three study groups. No significant changes in the thickness of the predentin tissue deposited in the region of the future cusp, in the middle third, nor the cervical third of the crown were observed in the three subgroups. The mineralized dentin had the same thickness in all three subgroups and in the crowns of the analyzed tooth germs. The length of the odontoblastic layer was not different between the fluoxetine-treated groups and the control group (Figure 5).
Discussion
The development of rat molars is a classical model to study odontogenesis. In a series of histological studies, the molars of rats have been analyzed for their reactions to various drugs and experimental conditions16.
Mammalian teeth represent a combination product during the development of the ectoderm and ectomesenchyme14. The influence of the oral epithelium on the mesoderm plays an important role in tooth germ development17.
No differences were found in the histological characteristics of the 1-day-old animals in any of the groups, i.e., the odontogenesis process began at the dental cusps that presented in a more advanced stage of dental crown development. An undifferentiated stage was observed down the slopes of the crowns to the cervical loop, as the ectomesenchymal dental papilla prepared for dentin formation. This process evolved gradually, and the dentin approached the cervical third of the crown in 5-days old animals. These data agree with the description of Beverlander and Hiroshi (1966)18 who reported that dentin begins to be formed in the advanced bell stage as a function of the dental papilla. The cells that differentiate in the periphery of the dental papilla are responsible for the formation and mineralization of the organic dentine matrix.
The odontoblasts are juxtaposed with the predentin layer and are present in greater numbers in the portion closer to the cusp19. Several sections showed pseudostratification of the odontoblast layer; however, this variation in tissue morphology can be explained by the difference in the angle of the thick and sloped region of the cusp in the tooth germ section. Moreover, the crown of a rat molar has a specific anatomy, with more than one cusp and deep grooves that contribute to formation of a larger number of bends15. These characteristics may be related to differences in thickness of the dentin matrix and the length of the odontoblast layer, depending on the selected region.
The odontoblasts synthesized the organic dentin matrix as dentinogenesis advanced, then retreated and left a cell extension that made contact with the basement membrane near the dentin-enamel junction. A pink band, which stained with less intensity, lay between the odontoblasts and dentin and stained more intensely in the 5-day-old animals, which characterized the deposition of predentin. These findings are consistent with results describing the presence of a matrix rich in sulfur and less acidophilic than the organic dentin matrix (predentin) in adjacent areas of the odontoblast layer20.
The dentin matrix stains acidophilic, acquiring a light pink hue when stained with hematoxylin and eosin, which indicates the presence of type-I collagen fibers and proteoglycans. These findings were reported by Reith (1968)21 who found that odontoblasts differentiate by location and size and are flanked by an extracellular compartment with collagen fibers and an amorphous substance.
In 1-day-old animals, the histometric data showed no significant differences in the thickness of the dentin matrix or the length of odontoblasts in any part of the tooth germ crown. Just as in the 5-day-old animals, all the parameters measured in histometric analysis were similar. As the mothers were treated only during pregnancy, fluoxetine administration did promote significant morphological and quantitative changes in those tissues. Even 5-day-old animals that had been breastfed and would receive fluoxetine through breast milk, showed no significant changes in tooth germ.
The choice of fluoxetine hydrochloride was justified for this study, because it is the most widely prescribed antidepressant worldwide. It is capable of inhibiting 5-HT reuptake4 and has little affinity for other neuroreceptors, which increases its tolerability by the body and diminishes side effects5.
Few studies have investigated use of SSRIs (fluoxetine hydrochloride) and the development of mineralized tissues that form the tooth, as most related studies investigated bone tissue. Bliziotes et al. (2006)9 found that 5-HT1A and 5-HT2A receptors in bone may be related to possible interactions with the serotonergic system.
A previous study found that 5-HT is present during morphogenesis of the craniofacial region; however, they did not discuss tooth development22. Other experimental evidence has also revealed that 5-HT may influence embryogenesis and growth23.
Battaglino et al. (2007)24 reported reduced cortical bone and trabecular bone mass in rats treated with fluoxetine, and concluded that fluoxetine inhibits normal bone growth in rats. Those authors claimed that 5-HT plays an important role in the differentiation of osteoblasts and osteocytes, and that interference of 5-HT by fluoxetine could reduce bone mass.
Bonnet et al. (2007)25 reported deleterious effects on the architecture, microarchitecture and biomechanics of bones in animals treated with fluoxetine hydrochloride. Similarly, Battaglino et al. (2004)10 concluded that fluoxetine inhibits the differentiation of osteoclasts derived from bone marrow cells.
Lauder et al. (2000)26 revealed that 5-HT2A, 5-HT2B, and 5-HT2C receptors in the developing tooth germ play a stimulating role during odontogenesis; however, they did not investigate development of the dentin-pulp complex.
Corroborating these findings, Moiseiwitsch, Lauder (1996)11 and Moiseiwitsch et al. (1998)12 analyzed the inhibitory effects that SSRIs have during organogenesis by culturing rat mandibles with different 5-HT concentrations. Those authors concluded that 5-HT could influence the dental papilla to induce the ameloblasts to differentiate from the external epithelium, inner epithelium, stellate reticulum, and stratum intermedium, which are essential tissues for growth and development of the tooth germ. However, they made no statements regarding dentinogenesis.
Cavalcanti et al. (2009)27 evaluated the morphological and embryonic developmental changes occurring in skeletal bones of animals whose mothers were treated with 10 mg/kg body weight fluoxetine hydrochloride. In the present study the temporomandibular joint was chosen because it is related to craniofacial development, which is influenced by the serotonergic system.
The findings of the present study were similar to those of Silva et al. (2010)28, who analyzed the direct effect of fluoxetine on the development of tooth enamel. In this study were evaluated the morphological and structural changes that this drug could produce in the enamel organ of the maxillary first molars of rats. The results showed no structural changes in the development of tissues that form the tooth enamel.
Thus, from these results, it may be suggested that fluoxetine hydrochloride administration during pregnancy, up to 20mg/kg, does not interfere on the dentin-pulp complex structural development. Further research is required with a different design and using a longer evaluation period to confirm the present data.
Acknowledgements
The financial support given by the National Council for Scientific and Technological Development (CNPq).
References
1. Morrison JL, Riggs KW, Rurak DW. Fluoxetine during pregnancy: impact on fetal development. Reprod Fertil Dev. 2005; 17: 641-50. [ Links ]
2. Patel SR, Wisner KL. Decision making for depression treatment during pregnancy and the postpartum period. Depress Anxiety. 2011; 28: 589-95. [ Links ]
3. Patil AS, Kuller JA, Rhee EH. Antidepressants in pregnancy: a review of commonly prescribed medications. Gynecol Surv. 2011; 66: 777-87. [ Links ]
4. Lanza di Scalea T, Wisner KL. Antidepressant Medication use during breastfeeding. Clin Obstet Gynecol. 2009; 52: 483-97. [ Links ]
5. Feijó FM, Bertoluci MC, Reis C. Serotonina e controle hipotalâmico da fome: uma revisão. Rev Assoc Med Bras. 2011; 57: 74-7. [ Links ]
6. Canto-de-Souza A, Souza RLN, Pelá IR, Graeff FG. Involvement of the midbrain periaqueductal gray 5-HT1A receptors in social conflict induced analgesia in mice. Eur J Pharmacol. 1998; 345: 253-6. [ Links ]
7. Buznikov GA, Lambert HW, Lauder JM. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001; 305: 177-86. [ Links ]
8. Lauder JM, Luo X, Persico AM. Serotonergic regulation of somatosensory cortical development: lessons from genetic mouse models. Devel Neurosci. 2003; 25: 173-83. [ Links ]
9. Bliziotes M, Eshleman A, Burt-Pichat B, Zhang XW, Hashimoto J, Wiren K, et al. Serotonin transporter and receptor expression in osteocytic MLO-Y4 cells. Bone. 2006; 39: 1313-21. [ Links ]
10. Battaglino R, Fu J, Spate U, Ersoy U, Joe M, Sedaghat L, et al. Serotonin regulates osteoclast differentiation through its transporter. J Bone Miner Res. 2004; 19: 1420-31. [ Links ]
11. Moiseiwitsch JRD, Lauder JM. Simulation of murine tooth development in organotypic culture by the neurotransmitter serotonin. Arch Oral Biol. 1996; 41:161-5. [ Links ]
12. Moiseiwitsch JRD, Raymond JR, Tamir H, Lauder JM. Regulation by serotonin of tooth-germ morphogenesis and gene expression in mouse mandibular explant cultures. Arch Oral Biol. 1998; 43: 789-800. [ Links ]
13. Lesot H, Brook AH. Epithelial histogenesis during tooth development. Arch Oral Biol. 2008; 19: 1-9. [ Links ]
14. Bei M. Molecular Genetics of Tooth Development. Curr Opin Genet Dev. 2009; 19: 504-10. [ Links ]
15. Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: structure, composition and mineralization. Front Biosci. 2011; 1: 711-35. [ Links ]
16. Pinzon RD, Kozlov M, Burch W. Histology of rat molar pulp at different ages. J Dent Res. 1967; 46: 202-8. [ Links ]
17. Thesleff I, Tummers M. Tooth organogenesis and regeneration. StemBook [internet]. Cambridge (MA): Harvard Stem Cell Institute; 2009.
18. Bevelander G, Hiroshi N. The formation and mineralization of dentine. Anat Rec. 1966; 156: 303-23. [ Links ]
19. Sasaki T, Garant PR. Structure and organization of odontoblasts. Anat Rec. 1996; 245: 235-49. [ Links ]
20. Goracci G, Mori G, Baldi M. Terminal end of the human odontoblasts process: a study using SEM and confocal microscopy. Clin Oral Investig. 1999; 3: 126-32. [ Links ]
21. Reith EJ. Collagen formation in developing molar teeth of rats. J Ultrastruct Res. 1968; 21: 383-414. [ Links ]
22. Reisoli E, De Lucchini S, Nardi I, Ori M. Serotonin 2B receptor signaling is required for craniofacial morphogenesis and jaw joint formation in Xenopus. Development. 2010; 137: 2927-37. [ Links ]
23. Bliziotes MM, Eshleman AJ, Zhang XW, Wiren KM. Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone. 2001; 29: 477-86. [ Links ]
24. Battaglino R, Vokes M, Schulze-Spate U, Sharma A, Graves D, Kohler T et al. Fluoxetine treatment increases trabecular bone formation in mice (Fluoxetine affects bone mass). J Cell Biochem. 2007; 100: 1387-94. [ Links ]
25. Bonnet N, Bernard P, Beaupied H, Bizot JC, Trovero F, Courteix D et al. Various effects of antidepressant drugs on bone microarchitectecture, mechanical properties and bone remodeling. Toxicol Appl Pharmacol. 2007; 221:111-8. [ Links ]
26. Lauder JM, Wilkie MB, Wu C, Singh S. Expression of 5-HT2A, 5-HT2B and 5-HT2C receptors in the mouse embryo. Int J Dev Neurosci. 2000; 8: 653-62. [ Links ]
27. Cavalcanti UDNT, Baratella-Evêncio L, Neto JE, Castro RM, Cardona AS, Melo MLM et al. Morphological aspects of the embryonic development of the TMJ in rats (Rattus norvegicus albinus) treated with fluoxetine. Int J Morphol. 2009; 27: 899-903. [ Links ]
28. Silva IHM, Leão JC, Evêncio LB, Porter SR, De Castro RM. Morphological analysis of the enamel organ in rats treated with fluoxetine. Clinics. 2010; 65: 61-6. [ Links ]
 Correspondence:
Correspondence:
Isabela Maria de Albuquerque Santiago
Avenida Prof. Moraes Rego, 1235
CEP: 50670-901, Recife, PE - Brasil
Phone/Fax: + 55 81 21268515
E-mail: isabelasantiago1@yahoo.com.br
Received for publication: November 25, 2012
Accepted: March 15, 2013













