Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.12 no.1 Piracicaba Jan./Mar. 2013
CASE REPORT
Differential diagnosis between post-polio syndrome symptoms and temporomandibular disorder - Clinical case
Gustavo Augusto Seabra Barbosa; Maria Helena de Siqueira Torres Morais
Department of Dentistry, Federal University of Rio Grande do Norte, Natal, RN, Brazil
ABSTRACT
Post-poliomyelitis syndrome (PPS) is characterized by the delayed appearance of new neuromuscular symptoms in patients several years after their acute poliomyelitis paralysis. Clinical features of PPS include fatigue, joint and muscle pain, new muscular weakness and bulbar symptoms. The diagnosis is essentially clinical after excluding other neurological, orthopedic or rheumatologic problems. Temporomandibular disorders (TMD) are usually diagnosed by means of comprehensive review of patient history and clinical examination and the symptoms are pain/ discomfort in the jaw, mainly in the region of the temporomandibular joints (TMJs) and/or masticatory muscles, limitation of mandibular function and/or TMJ sounds. In the same way as PPS, the diagnosis of TMD is challenging. This study reports the case of a patient that presented the symptoms of both conditions in the stomatognathic system, and discusses how to achieve the differential diagnosis for proper management of the cases.
Keywords: diagnosis differential, post-poliomyelitis syndrome, temporomandibular disorders.
Introduction
In the first half of the 20th century, poliomyelitis was greatly feared. It often struck without warning, was highly contagious, and affected large, young populations, causing prolonged or permanent flaccid paralysis or death1. Transmission can occur from person-to-person through nasopharyngeal secretions or of objects, food and water, contaminated with feces of patients or carriers2. This disease is caused by viral damage of motor neurons in the spinal cord. Loss of muscle fibers is furthermore compensated through hypertrophy of the remaining muscle fibers3. The onset of spinal poliomyelitis is associated with myalgia and severe muscle spasms, with the subsequent development of an asymmetrical, predominantly lower limb, flaccid weakness that becomes maximal after 48 h2. After the introduction of vaccination in the 1950s, the epidemics disappeared from most countries and polio became a rare disease2-3.
Patients with polio may experience progression with new symptoms decades after the acute disease. These late symptoms are termed post-polio syndrome (PPS)3-5 and this designation was introduced by Halstead and Rossi in 1985 apud Ramaj (2007)6. The nature of the condition remains controversial and diagnosis is essentially clinical after excluding other neurological, orthopedic or rheumatologic problems. Weakness with generalized fatigue is the most common symptom, but joint pain, muscle pain, atrophy, cold intolerance, respiratory insufficiency and dysphagia may also be present1,3,5-6. PPS occurs 30 to 40 years after an acute poliomyelitis attack and is observed in approximately 25 to 28% of patients and according to the visual analogue scale (VAS) the intensity of pain is relatively high5. Current diagnosis is based on thorough clinical examinations in order to eliminate other possible diagnosis7 but it is still unclear at this point time if the occurrence of PPS increases with age1,3-4,6,s. To date, several risk factors for PPS development have been reported, although the etiology of this disorder remains elusive9. The early detection of correctable and treatable causes of late-onset weakness and pain may help to reduce the functional declines of polio survivors10.
The term "temporomandibular disorders" (TMD) has been used as a collective term that involve the masticatory musculature, the TMJs and associated structures, or both. These disorders have been identified as a major cause of nondental pain in the orofacial region, and are considered to be a subclassification of musculoskeletal disorders11-13. In general, TMD mostly appear in the adult popution14. Orofacial pain and TMD can be associated with pathologic conditions or with disorders related to somatic and neurologic structures, such as primary headache disorders and rarely have a solitary cause and numerous factors have been implicated15. This is further compounded by the patient habitually reporting several problems during history taking and clinical examination.15. A complete understanding of the associated medical conditions with symptoms common to TMD and orofacial pain is necessary for a proper diagnosis13.
In view of difficult of diagnosis and the similar symptoms between PPS and TMD, this paper reports the case of a patient that presented symptoms of both entities in the stomatognathic system, and discusses how to achieve the differential diagnosis for proper management of the cases.
Clinical Case
A 42-year-old Caucasian female patient sought the Department Of Dentistry of the Federal University of Rio Grande do Norte (UFRN, Brazil) complaining of fatigue in facial muscles, clicking sounds and pain in the region near the TMJ, and "an unbearable weight on the face". By exclusion of orthopedic, neurological and rheumatic problems, these symptoms were attributed to PPS, as the patient that had contracted polio at the age of 1 year and 10 months.
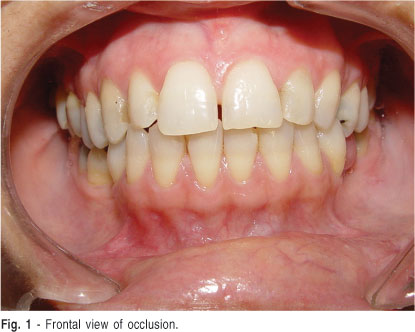
During the clinical interview, the patient reported tooth clenching, which was confirmed by the present of wear facets on several teeth (Figure 1). The clinical examination also revealed a clicking sound on mouth opening/closure at the left TMJ and tenderness to palpation of the lower portion of the left lateral pterygoid muscle and masseter muscles of the both sides. The diagnosis was suggestive of disk displacement with reduction in the left TMJ accompanied by myofascial pain. A visual analogue scale for pain (VAS), usually used to check the effectiveness of the proposed treatment, was applied and the patient reported pain level 5 on the scale from zero to 10.
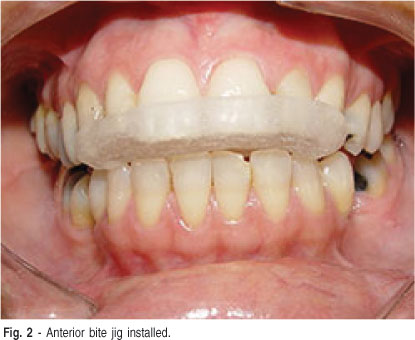
We suspected that the parafunctional habit was causing the muscle and TMJ pain instead of the PPS, and this was informed to the patient. In order to clarify this point, the patient wore an anterior bite jig during 3 intercalated days for neuromuscular deprogramming (Figure 2). The patient reported immediate relief after installation of the device (VAS = 0), and thus the use of a stabilization occlusal splint associated with behavioral therapy was prescribed.
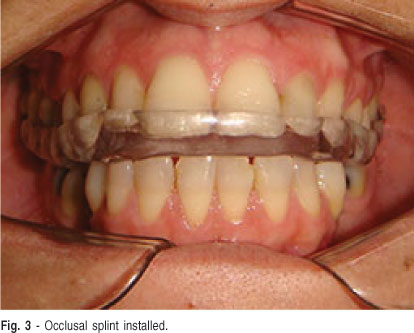
Upper and lower plaster models (Durone IV, Dentsply) were obtained from alginate impressions (Jeltrate Plus, Dentsply) and mounted in semi adjustable articulator. The upper model was mounted using the facial arc. Using the neuromuscular deprogramming device16, registration was taken with vinyl polysiloxane (Occwfast, Zhermack) in centric relation to mount the lower model. The oclusal splint was waxed and fabricated using conventional thermally activated acrylic resin (Classico, Art. Odontológicos Classico). The contacts of teeth with the oclusal splint were adjusted in centric relation occlusion. The lateral and anterior excursive movements were adjusted with the canine and anterior guides, respectively. The patient was instructed to use the stabilization occlusal splint during sleep and avoid the parafunctional habit while awake (Figure 3). The supposed TDM etiology was explained to the patient in order to reduce the repetitive strain of the masticatory system (tooth clenching), to encourage relaxation, and to control the amount of the masticatory activity.
There was a significant improvement in painful symptoms 1 week after installation of the occlusal splint. The patient reported pain level zero in VAS, decreased fatigue and relief in the TMJ tenderness. After 1-year of treatment, the patient was free of symptoms in the facial region.
Discussion
PPS presents as a new onset of weakness, fatigue, fasciculations, and pain with additional atrophy of the muscle group involved during the initial paralytic disease 20 or more years earlier. This syndrome is more common among women and after longer time of the acute disease1,3-7. The pathogenesis of PPS is not completely understood. The most widely accepted hypotheses suggests a dysfunction of surviving motor neurons that causes a progressive loss of the terminals on single axons or a dysfunction of motor units that are already weak, owing to forced exercise1-2,10.
The current diagnosis for PPS was first described by Mulder et al. 4 in 1972 apud Lin. These criteria are 1) a prior episode of poliomyelitis with residual motor neuron loss; 2) a period of at least 15 years of neurological and functional stability after recovery from the acute illness; 3) gradual or, rarely, abrupt onset of new weakness or abnormal muscle fatigue; and 4) the exclusion of other conditions that could cause similar manifestations. When no alternative explanation can be found, this late onset weakness is referred to as PPS1-2,4.
The combined effect of aging, overwork, weight gain, other medical comorbidities, and muscular overuse or disuse play a role in new weakness, pain and fatigue. It is important to note that there is no reactivation of the original poliomyelitis virus or reinfection. This is often a specific anxiety in PPS patients and needs to be addressed3,11.
Prospective studies have focused mainly on the progression of neurologic deficit, in terms of loss of muscle strength. Although results are sometimes conflicting, they do not suggest a rapid loss of muscle force over time. In contrast, relatively little attention has yet been paid to functional assessment in PPS, which is surprising since the loss of functional abilities is probably a major concern to the involved patients6.
There is no way for preventing late symptoms and little is known to which extent rehabilitation influences the long-term outcome4.
Symptoms usually appear earlier in patients who have a lot of residual weakness, early bulbar respiratory difficulty during the acute illness, and those who were older when they contracted acute polio. Patients suffer from pain in the joints, bones and muscles. They also have fatigue with muscle wasting, weakness, cramps and fasciculation. There is a severe deterioration in functional abilities including mobility and activities of daily living4,6.
The TMD symptoms are well known. They include pain/ discomfort in the jaw, mainly in the region of the TMJs and/ or muscles of mastication, limitation of mandibular function and/or TMJ sounds11,17 However, a correct diagnosis is frequently very difficult. Our patient presented both the PPS symptoms 40 years after polio and the TMD symptoms.
Behavioral therapy is generally considered as a first conservative approach for the treatment of TMD patients18. There is an application of behavioral science theories and methods to change the perception and appraisal of pain and to attenuate or eliminate the personal suffering and psychosocial dysfunction that often accompanies persistent pain conditions. The rationale for choosing behavioral therapy arises from the idea that parafunctional activity and psychosocial factors play a role in the pathogenesis of musculoskeletal pain. This method has proved to be effective in TMD management18-19. Furthermore the patient compliance is of fundamental importance. Compare to others treatments, the occlusion splint is much less demanding on the patients and some of them prefer this modality20.
The underlying concept of the anterior bite jig is to deprogram the memorized pattern of muscle activity by preventing tooth contacts at the moment of swallowing. It is presumed to work through a relaxation effect, leading to a reduction in the muscle activity at postural position13. This device was used for differential diagnosis. The patient reported pain level zero in VAS=0 using it.
Splint therapy is a non-invasive and reversible biomechanical method of managing pain and dysfunction of the craniomandibular articulation and its associable musculature20-24. Why occlusal splints are effective in reducing symptoms is not entirely clear, but several theories have been proposed to accountant for their mechanisms of action21,23. Dylina (2001)25 reported that the splints have at least 6 functions, including the following: to relax the muscles, to allow the condyle to seat in the centric relation occlusion, to provide diagnostic information, to protect teeth and associated structures from bruxism, to mitigate periodontal ligament proprioception, and to reduce cellular hypoxia levels25. The characteristics of a successful splint should include occlusal stability, equal intensity stops on all teeth; immediate posterior disocclusion during excursion movements; smooth transitions in lateral, protrusive, and extended lateral excursions (crossover); comfort during wear; and reasonable esthetics. Patient compliance also contributes to splint therapy success25.
Splint therapy can be an important diagnostic tool to determine TMD status. If a patient rapidly becomes comfortable with a splint, it may be an indication that the disorder is of muscular origin. If symptoms worsen with permissive splint wear, this may indicate an internal derangement (disk) problem (perhaps caused by free reign of the condylar head back to the retrodiscal tissues without housing by the disk) or an error in the initial diagnosis25.
Using the splint, our patient remained without symptoms. It can assign the absence of pain due to possible effects of the splint as muscle relaxation21, changes in impulses to the central nervous system (CNS)26 and cognitive theory11 (according to this theory, the presence of splint as a foreign object in the mouth would likely change the oral tactile stimuli, decrease the oral volume and space for the tongue, and make the patients conscious about the position and potentially harmful use of their jaw).
As described, the clinical diagnosis of PPS is made by exclusion of options and requires differentiation with other diseases that may present similar characteristics; however, in the present case, there was probably no exclusion of TMD for symptoms in the facial region.
PPS and TMD are difficult to diagnose because the symptoms are non-specific. PPS is essentially clinical after excluding other neurological, orthopedic or rheumatologic problems. In most cases, the diagnosis of TMD is based on careful review of patient history and clinical examination, which depends on patient report of levels of pain/discomfort at the TMJs and associated muscles. Without a differential diagnosis the two entities may be confused due to their similarities.
In conclusion, this clinical report describes an association, but not necessarily a cause-effect relationship between PPS and TMD symptoms. More specific investigations are necessary for a better understanding of a possible link between PPS and TMD.
References
1. Howard RB. Poliomyelitis and the postpolio syndrome. BMJ. 2005; 330: 1314-8. [ Links ]
2. Vranjac, A. Poliomyelitis and the postpolio syndrome. Sao Paulo: State Department of Health; 2006. [ Links ]
3. Nollet F, Beelen A, Prins MH, de Visser M, Sargeant AJ, Lankhorst GJ et al. Disability and functional assessment in former polio patients with and without postpolio syndrome. Arch Phys Med Rehabil. 1999; 80: 136-43. [ Links ]
4. Lin KH, Lim YW. Post-poliomyelitis Syndrome: case report and review of the literature. Ann Acad Med Singapure. 2005; 34: 447-9. [ Links ]
5. Werhagen L, Borg K. Impact of pain on quality of life in patients with postpolio syndrome. J Rehabil Med. 2013: 45: 161-3. [ Links ]
6. Ramaraj R. Post Poliomyelitis syndrome: clinical features and management. Br J Hosp Med. 2007; 68: 648-50. [ Links ]
7. Gonzalez H, Khademi M, Borg K. Intravenous immunoglobulin treatment of the post-polio syndrome: sustained effects on quality of life variables and cytokine expression after one year follow up. J Neuroinflammation 2012; 9: 1-12. [ Links ]
8. Matyja E. Post-polio syndrome. Part I. The "legacy" of forgotten disease, challenges for professionals and polio survivors. Neurol Neurochir Pol. 2012; 46: 357-71. [ Links ]
9. Bertolasi L, Acler M, dall'Ora E, Gajofatto A, Frasson E, Tocco P et.al. Risk factors for post-polio syndrome among an Italian population: a case-control study. Neurol Sci. 2012; 33: 1271-5. [ Links ]
10. Lim JY, Kim KE, Choe G. Myotonic Dystrophy Mimicking Postpolio Syndrome in a Polio Survivor. Am J Phys Med Rehabil. 2009; 88: 161-5. [ Links ]
11. Suvinen T, Reade PC, Kemppainen P, Könönen M, Dworkin SF. Review of aetiological concepts of temporomandibular pain disorders: towards a biopsychosocial model for integration of physical disorder factors with psychological and psychosocial illness impact factors. Eur J Pain. 2005; 9: 613-33. [ Links ]
12. Al-Ani Z,Gray R. TMD Current Concepts: 1. An Update. Dent Update. 2007; 34: 278-88. [ Links ]
13. Auvenshine RC. Temporomandibular Disorders: Associated Features. Dent Clin N Am. 2007; 51: 105-27. [ Links ]
14. Niemela K, Korpela M, Raustia A, Ylöstalo P, Sipilä K. Efficacy of stabilisation splint treatment on temporomandibular disorders. J Oral Rehabil. 2012; 39: 799-804. [ Links ]
15. Jerjes W. Muscle disorders and dentition-related aspects in temporomandibular disorders: controversies in the most commonly used treatment modalities Int Arch Med. 2008; 23: 1-13. [ Links ]
16. Lucia VO. A technique for recording centric relation. J Dent. 1964; 14: 492505. [ Links ]
17. Pimentel MJ, Gui MS, Martins de Aquino LM, Rizzatti-Barbosa CM. Features of temporomandibular disorders in fibromyalgia syndrome. Cranio. 2013; 31: 40-5. [ Links ]
18. Michelotti A, Wijer ,Steenks M, Farella M. Home-exercise regimes for the management of non-specific temporomandibular disorders. J Oral Rehabil. 2005; 32: 779-85. [ Links ]
19. Dworkin S. Behavioral and educational modalities. Oral Surg Oral Med Oral Pathol. 1997; 8: 128-33. [ Links ]
20. De Felicio CM, Melchior MO, Silva MAM. Effects of orofacial myofunctional therapy on temporomandibular disorders. Cranio. 2010; 28: 250-61. [ Links ]
21. Pertes RA, Gross SG. Clinical management of temporomandibular disorders and orofacial pain. Chicago: Quintessence; 2005. p. 197-209. [ Links ]
22. Major PW, Nebbe B. Use and effective of splint appliance therapy: review or literature. Cranio. 1997; 15: 159-66. [ Links ]
23. Nelson SJ. Principles of stabilization bite splint therapy. Dent Clin North Am. 1995; 39: 403-21. [ Links ]
24. Lindfors E, Magnusson T, Teglberg A. Interocclusal appliances- Indications and clinical routines in general dental practice in Sweden. Swed Dent J. 2006; 30: 123-34. [ Links ]
25. Dylina TJ. A common-sense approach to splint therapy. JProsthet Dent. 2001; 86: 539-45. [ Links ]
26. Okeson JP. Management of temporomandibular disorders and occlusion. 6th ed. Saint Louis: Mosby; 2008. p. 377-99. [ Links ]
 Correspondence:
Correspondence:
Gustavo Augusto Seabra Barbosa
Av. Salgado Filho, 1787, Lagoa Nova
CEP: 59056-000 - Natal, RN, Brasil
Phone: 55 84 32154135
E-mail: gustavoseabra@hotmail.com
Received for publication: March 19, 2012
Accepted: August 11, 2012













