Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.12 no.1 Piracicaba Jan./Mar. 2013
CASE REPORT
Combined therapy with mineral trioxide aggregate, and guided tissue regeneration for a large radicular cyst: a 13-year follow-up
Pedro Felício Estrada Bernabé; João Eduardo Gomes-Filho; Eloi Dezan-Júnior; Annelise Katrine Carrara Prieto; Renata Oliveira Samuel; Luciano Tavares Angelo Cintra
Department of Endodontics, Araçatuba School of Dentistry, UNESP - Univ Estadual Paulista, Araçatuba, SP, Brazil
ABSTRACT
Biomaterials such as membrane barriers and/or bone grafts are often used to enhance periapical new bone formation. A combination of apical surgery and these biomaterials is one of the latest treatment options for avoiding tooth extraction. In case of periapical lesions, guided tissue regeneration (GTR) is attempted to improve the self-regenerative healing process by excluding undesired proliferation of the gingival connective tissue or migration of the oral epithelial cells into osseous defects. In many cases, GTR is necessary for achieving periodontal tissue healing. This report describes the healing process after surgery in a challenging case with a long-term follow-up. In this case report, endodontic surgery was followed by retrograde sealing with mineral trioxide aggregate (MTA) in the maxillary right central incisor and left lateral incisor. Apicectomy was performed in the maxillary left central incisor and a 1-mm filling was removed. The bone defect was filled with an anorganic bone graft and covered with a decalcified cortical osseous membrane. No intraoperative or postoperative complications were observed. After 13 years of follow-up, the patient showed no clinical signs or symptoms associated with the lesion and radiographic examination showed progressive resolution of radiolucency. In conclusion, the combination of apical surgery and regenerative techniques can successfully help the treatment of periapical lesions of endodontic origin and is suitable for the management of challenging cases.
Keywords: guided tissue regeneration, apical surgery, MTA.
Introduction
Radicular cysts are common inflammatory cystic lesions that develop in the apical tissues as consequence of an infected and necrotic pulp1. Although in most cases small cystic lesions heal after endodontic therapy, in case of larger lesions, additional treatment may be needed2. Apical surgery for radicular cysts generally involves apical root resection and sealing with endodontic material3.
Currently, the preferred root-end filling material is mineral trioxide aggregate (MTA) because it has some biological properties, such as induction of calcification that enables biological sealing4-6. The physiochemical and biological properties of MTA have been reported in numerous papers4-7. However, the ideal scenario would be to improve the benefits offered by the MTA with the aid of other techniques that promote tissue regeneration.
Retrospective studies have shown that the success rate of apical surgery is not as high as expected8-9. Apical surgery was considered unsuccessful in about 1 out of every 4 cases in 1960-197910. From the 1980s, the success rates increased to 50%, and the sizes of the lesions reduced in more than 25% of the cases11. However, the success rate of surgery remains low in cases of endoperiodontal lesions8. The high failure rate of apical surgery is directly related to the variety of factors that can influence the healing process in the periapical region7,9. Adequate results were obtained in studies related to the healing process and the regeneration of tissues, especially in the ones pertaining to the support and protection of the periapex12-13. These results were obtained by developing regenerative and reparative techniques that helped to reestablish the periodontal structures and to preserve the biological width of the involved tissues14.
Use of guided tissue regeneration (GTR) in apical surgery can increase the success rate of this procedure15. The technique helps creating ideal conditions for the restoration of original structures and normal functioning of the tissues that were lost because of infectious and inflammatory processes16. The basic principle of GTR is cellular selectivity. The technique aims at enhancing the quality and quantity of new bone and accelerating bone growth around the bone cavity17. The barrier is put on the bone defect and may frequently be associated with osseous grafting materials. This avoids the penetration of cells from both the epithelial tissue and gingival connective tissue. The use of the barrier membrane affords the time needed for the differentiation, proliferation and migration of the cells from the ligament, and from periodontal and alveolar bones to the bone cavity, favoring the healing process. Furthermore, the space created by the membrane enables undifferentiated mesenchymal cells to migrate to this area and differentiate, thus promoting osteogenesis without the interference of other types of competitor cells12-13.
The aim of the present case report was to describe a clinical situation in which combined therapy with MTA and GTR was performed to treat a large radicular cyst.
Case Report
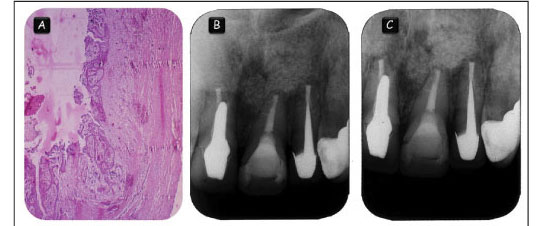
A 45-year-old woman who had previously undergone endodontic treatment for apical lesions associated with the maxillary left central incisor sought treatment at the Apical Surgery Center at the Araçatuba School of Dentistry at the UNESP. The patient presented poorly adapted prostheses in the maxillary right central incisor and left lateral incisor, as well as a large resin restoration in the maxillary left central incisor (Figure 1A). Radiographic examination revealed extensive apical lesions associated with the maxillary right and left central incisors and the left lateral incisor, in which root canal treatment had failed (Figure 1B).
The first suggested treatment option was the removal of the crowns/posts and retreatment of the maxillary right central incisor and left lateral incisor. However, the patient refused the proposed treatment due to the risks involved and opted for apical surgery. Complementary laboratory exams showed that the patient had no systemic alteration. Prophylactic antibiotics were prescribed 1 h prior to the surgery.
The surgical area was disinfected with iodine solution and 0.12% chlorhexidine gluconate. Prilocaine hydrochloride (3%) with octapressin (Dentsply, Petrópolis, RJ, Brazil) was used for local anesthesia. The flap design consisted of 2 releasing incisions connected by a sulcular incision (Figure 1C). The apical lesion was removed with size 85 Lucas surgical curettes (Hu-Friedy, Chicago, IL, USA) and size 35 and 36 curettes (Dentsply Maillefer, Tulsa, OK, USA) (Figure 1D). Apical roots were sectioned (3-mm sections) perpendicular to the long axis of the root with a Zekrya bur (Dentsply Maillefer) with a high-speed handpiece (Figure 1E). Different treatment approaches were performed for each tooth.
For the maxillary central right incisor, retrograde endodontic treatment was performed using pre-bent files with a 4 mm length and filled with Pro-Root MTA® (Dentsply Maillefer). Apicectomy and removal of the apical filling (1 mm) was performed in the maxillary central left incisor because it was properly treated. For the maxillary lateral left incisor, retrograde endodontic treatment was performed using 6 mm pre-bent files and Pro-Root MTA® as the root-end filing material. Pro-Root MTA® was prepared according to the manufacturer's instructions and inserted using the MAP System device (Produits Dentaires, Vevey, Switzerland).
After the surgical procedures, radiographs were taken to verify the quality of the root-end treatments. The bone defect was filled with an anorganic bone graft (Gen-Ox; Genius, São José dos Campos, SP, Brazil) (Figure 1F-H) and covered with a decalcified cortical osseous membrane (Gen-Derm; Genius, São José dos Campos, SP, Brazil) (Figure Finally, the flap was sutured with a simple interrupted suture using 4.0 silk (Ethicon, São José dos Campos, SP, Brazil) (Figure 1J). After the surgery, the patient received antibiotics and medication to control pain.
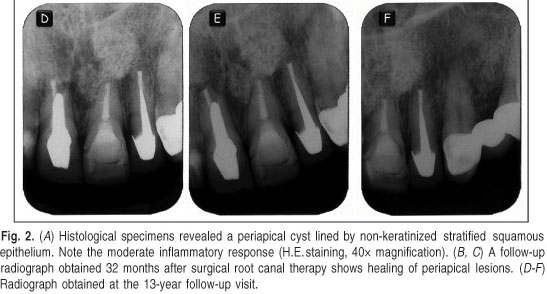
A full histological study of the cystic capsule was performed to confirm the previous diagnostic hypothesis (Figure 2A). After 7 days, the sutures were removed and the patient was examined. The patient experienced no pain and showed no swelling. No intraoperative or postoperative complications were observed. At the 32-month follow-up, the tooth had no clinical signs or symptoms and radiographic examination showed progressive resolution of radiolucency (Figure 2B, C).
A follow-up evaluation performed after 13 years confirmed clinical silence and normal apical radiographic aspects (Figure 2D-F). It was also verified that the patient had not changed the prosthetic crowns, preserving the posts, following the recommendations after surgery.
Discussion
Radicular cysts are also known as periapical cysts, dental cysts or apical periodontal cysts. A radicular cyst is generally asymptomatic, grows slowly and rarely grows large enough to erode extensively the adjacent bone structures. Enucleating the cyst is one of the recommended treatments1, but surgical procedures alone are not sufficient for a successful treatment of radicular cysts. One of the main goals of conventional endodontic treatment is to prevent the invasion of bacteria and their byproducts from the root canal system into the periradicular tissues of teeth in cases of apical periodontitis18.
The use of GTR techniques has been proposed as an adjunct to endodontic surgery to favor bone healing19. GTR has been accepted as a viable treatment for gingival recession20, intrabone defects21-22, vertical ridge augmentation23, furcation defects24, circumferential periodontal and dental implant-associated defects25-26, and in apical microsurgery12,18.
Membrane barriers and/or bone graft materials have also been used in periapical surgery to enhance new bone formation3. However, there are significant differences in the application of GTR between periodontal regenerative therapy and apical surgery. Regeneration represents replacement of damaged tissue by the cells of the same tissue. Repair occurs when the healing process results in the formation of new tissue with cells and structures that have the ability to behave differently from the original ones18. In apical surgery, the resected root end cannot be regenerated. Complete periapical wound healing after periapical surgery includes regeneration of the alveolar bone, periodontal ligament and cementum27. The application of a membrane barrier and/or bone graft during periapical surgery may not result in a complete regeneration of the apical tissues3. The adequate apical healing would be deposition of the cementum on the resected surface and root-end filling material4-6 and re-establishment of the biological width and periodontal ligament12-13.
In this case report, a large bone defect involving 3 teeth was detected and the use of GTR was justified. In a previous study it was observed that the use of biomaterials such as GenOx® combined with the GenDerm® membrane provided better results than blood clot for treating critical-size defects28. The distance between the margins of the bone cavity determined the type of cells that would first migrate to the defect site and consequently the tissue that would be formed8. Fibrous scar formation may occur in cases in which GTR is not used. The membranes used in apical surgeries, which may or may not be in contact with bone graft materials, have the special function of guiding the formation of the new bone in the apical defect and may enhance the healing process. The bone graft material used in this case was an anorganic bovine bone that participated in the development of the new bone tissue and can act as an osteoconductive material.
In the present case, a retrograde, not direct, endodontic retreatment was performed for the maxillary central right incisor and left lateral incisor because of the presence of the prostheses. On the other hand, for the maxillary left central incisor, which had been subjected to endodontic treatment recently, a curettage was performed followed by apicoectomy and remodeling of the filling 1 mm from the apex.
In this case, the lesions had a positive response to the combination of surgical treatment and biomaterials. The use of membrane barriers and other agents, such as bone graft materials or tissue growth factors, has been reported as a viable treatment option1,3,27-28. No intraoperative or postoperative complications were observed. At the 13-year follow-up, the patient showed no clinical signs or symptoms associated with the lesion and radiography showed progressive resolution of the radiolucency.
Compared to the traditional methods of endodontic surgery, GTR techniques have significantly improved the outcomes for periapical lesions29. A review of literature suggests that there is a lot of optimism about regenerative procedures. However, despite the success achieved with these procedures, as seen in this case report, they should be applied with caution. Biological studies in experimental models should be conducted to evaluate the need for GTR use with apical surgery.
References
1. Sagit M, Guler S, Tasdemir A, Akf Somdas M. Large radicular cyst in the maxillary sinus. J Craniofac Surg. 2011; 22: e64-5. [ Links ]
2. Martin SA. Conventional endodontic therapy of upper central incisor combined with cyst decompression: a case report. J Endod. 2007; 33: 753-7. [ Links ]
3. Lin L, Chen MY, Ricucci D, Rosenberg PA. Guided tissue regeneration in periapical surgery. J Endod. 2010; 36: 618-25. [ Links ]
4. Cintra LT, de Moraes IG, Estrada BP, Gomes-Filho JE, Bramante CM, Garcia RB et al. Evaluation of the tissue response to MTA and MBPC: Microscopic analysis of implants in alveolar bone of rats. J Endod. 2006; 32: 556-9. [ Links ]
5. Gomes-Filho JE, de Moraes Costa MT, Cintra LT, Lodi CS, Duarte PC, Okamoto R et al. Evaluation of alveolar socket response to Angelus MTA and experimental light-cure MTA. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 110: e93-7. [ Links ]
6. Gomes-Filho JE, de Moraes Costa MM, Cintra LT, Duarte PC, Takamiya AS, Lodi CS et al. Evaluation of rat alveolar bone response to Angelus MTA or experimental light-cured mineral trioxide aggregate using fluorochromes. J Endod 2011; 37: 250-4. [ Links ]
7. Bernabé PF, Gomes-Filho JE, Rocha WC, Nery MJ, Otoboni-Filho JA, Dezan-Júnior E. Histological evaluation of MTA as a root-end filling material. Int Endod J. 2007; 40: 758-65. [ Links ]
8. Douthitt JC, Gutmann JL, Witherspoon DE. Histologic assessment of healing after the use of a bioresorbable membrane in the management of buccal bone loss concomitant with periradicular surgery. J Endod. 2001; 27: 404-10. [ Links ]
9. Song M, Jung IY, Lee SJ, Lee CY, Kim E. Prognostic factors for clinical outcomes in endodontic microsurgery: a retrospective study. J Endod. 2011; 37: 927-33. [ Links ]
10. Rud J, Andreasen JO, Jensen JE. A follow-up study of 1,000 cases treated by endodontic surgery. Int J Oral Surg. 1972; 1: 215-28. [ Links ]
11. Gutman J, Harrison J. Surgical endodontics. Cambridge: Blackwell Scientific; 1991. p.355-7. [ Links ]
12. Britain SK, Arx T, Schenk RK, Buser D, Nummikoski P, Cochran DL. The use of guided tissue regeneration principles in endodontic surgery for induced chronic periodontic-endodontic lesions: a clinical, radiographic, and histologic evaluation. J Periodontol. 2005; 76: 450-60. [ Links ]
13. Von Arx T, Britain S, Cochran DL, Schenk RK, Nummikoski P, Buser D. Healing of periapical lesions with complete loss of the buccal bone plate: a histologic study in the canine mandible. Int J Periodontics Restorative Dent. 2003; 23: 157-67. [ Links ]
14. Nandlal B, Daneswari V. Restoring biological width in crown-root fracture: a periodontal concern. J Indian Soc Pedod Prev Dent. 2007; 25 (Suppl): S20-4. [ Links ]
15. Taschieri S, Corbella S, Tsesis I, Bortolin M, Del Fabbro M. Effect of guided tissue regeneration on the outcome of surgical endodontic treatment of through-and-through lesions: a retrospective study at 4-year follow-up. Oral Maxillofac Surg. 2011; 15: 153-9. [ Links ]
16. Gagnon K, Morand MA. [Guided tissue regeneration in endodontics. [Part 1]. J Can Dent Assoc. 1999; 65: 394-8.
17. Pecora G, Baek SH, Rethnam S, Kim S. Barrier membrane techniques in endodontic microsurgery. Dent Clin North Am. 1997; 41: 585-602. [ Links ]
18. Artzi Z, Wasersprung N, Weinreb M, Steigmann M, Prasad HS, Tsesis I. Effect of guided tissue regeneration on newly formed bone and cementum in periapical tissue healing after endodontic surgery: an in vivo study in the cat. J Endod. 2012; 38: 163-9. [ Links ]
19. Taschieri S, Del Fabbro M, Testori T, Saita M, Weinstein R. Efficacy of guided tissue regeneration in the management of through-and-through lesions following surgical endodontics: a preliminary study. Int J Periodontics Restorative Dent 2008; 28: 265-71. [ Links ]
20. Rosetti EP, Marcantonio RA, Cirelli JA, Zuza EP, Marcantonio E Jr. Treatment of gingival recession with collagen membrane and DFDBA: a histometric study in dogs. Braz Oral Res. 2009; 23: 307-12. [ Links ]
21. Arikan F, Becerik S, Sonmez S, Gurhan I. Effect of platelet-rich plasma on gingival and periodontal ligament fibroblasts: new in-vitro growth assay. Braz J Oral Sci. 2007; 6: 1432-7. [ Links ]
22. Kaur M, Ramakrishnan T, Amblavanan N, Emmadi P. Effect of platelet-rich plasma and bioactive glass in the treatment of intrabony defects - a split-mouth study in humans. Braz J Oral Sci. 2010; 9: 108-14. [ Links ]
23. Rothamel D, Schwarz F, Herten M, Ferrari D, Mischkowski RA, Sager M, et al. Vertical ridge augmentation using xenogenous bone blocks: a histomorphometric study in dogs. Int J Oral Maxillofac Implants. 2009; 24: 243-50. [ Links ]
24. Sallum EA, Pereira LSS, Caffesse RG, Nociti FH, Casatti MZ, Sallum, AW. GTR in class III furcation defects with resorbable polylactic acid membranes. A histomorphometric study in dogs. Braz J Oral Sci. 2002; 1: 76-83. [ Links ]
25. Markou N, Pepelassi E, Kotsovilis S, Vrotsos I, Vavouraki H, Stamatakis HC. The use of platelet-rich plasma combined with demineralized freeze-dried bone allograft in the treatment of periodontal endosseous defects: a report of two clinical cases. J Am Dent Assoc. 2010; 141: 967-78. [ Links ]
26. Grunder U, Wenz B, Schupbach P. Guided bone regeneration around single-tooth implants in the esthetic zone: a case series. Int J Periodontics Restorative Dent. 2011; 31: 613-20. [ Links ]
27. Tsesis I, Rosen E, Tamse A, Taschieri S, Del Fabbro M. Effect of guided tissue regeneration on the outcome of surgical endodontic treatment: a systematic review and meta-analysis. J Endod. 2011; 37: 1039-45. [ Links ]
28. Bernabé PF, Melo LG, Cintra LT, Gomes-Filho JE, Dezan Jr E, Nagata MJ. Bone healing in critical-size defects treated with either bone graft, membrane, or a combination of both materials: a histological and histometric study in rat tibiae. Clin Oral Implants Res. 2012; 23: 384-8. [ Links ]
29. Kahler B. Microsurgical endodontic retreatment of a maxillary molar with a separated file: a case report. Aust Dent J. 2011 Mar; 56(1): 76-81. [ Links ]
 Correspondence:
Correspondence:
Luciano Tavares Angelo Cintra
Araçatuba School of Dentistry
São Paulo State University
Rua José Bonifácio, 1193, Araçatuba, SP, Brasil
Phone: +55 18 36363252 Fax: +55 18 36363279
E-mail:lucianocintra@foa.unesp.br
Received for publication: October 25, 2012
Accepted: December 11, 2012