Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.12 no.2 Piracicaba Abr./Jun. 2013
ORIGINAL ARTICLE
Is bleeding on probing a differential diagnosis between periimplant health and disease?
Priscila Ladeira CasadoI; Ricardo Villas-BôasII; Luana Cristine Leão da SilvaIII; Cristiana Farias de Carvalho AndradeIII; Letícia Ladeira BonatoIV; José Mauro GranjeiroV
IArea of Morphology, Cell Therapy Center - Clinical Research Unit and Biology Institute, Fluminense Federal University – Niterói, RJ, Brazil and Orthopedics and Traumatology National Institute, Rio de Janeiro, RJ, Brazil
IIArea of Dentistry, Veiga de Almeida University, Rio de Janeiro, RJ, Brazil
IIIDentist, Veiga de Almeida University, Rio de Janeiro, RJ, Brazil
IVDentist, Juiz de Fora Federal University, Juiz de Fora, MG, Brazil
VArea of Chemistry, Cell Therapy Center - Clinical Research Unit and Biology Institute - Fluminense Federal University - Niterói, RJ, Brazil and National Institute of Metrology - Rio de Janeiro, RJ, Brazil
ABSTRACT
As far as the periimplant anatomy is considered, the question raised is whether or not healthy periimplant tissues present bleeding on probing (BOP).
AIM: To assess if the criterion BOP is strictly related to periimplant disease (PID).
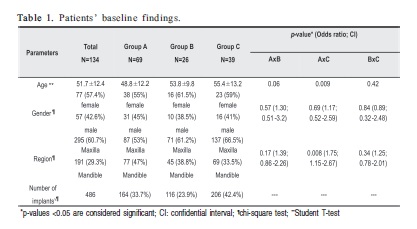
METHODS: 134 patients were included in this study. All periimplant regions were clinically and radiographically evaluated. Patients were assigned to 3 groups based on radiographic and clinical aspects in the periimplant region: Group A (healthysites) - no signs of mucosal inflammation or bone loss; Group B (mucositis) - red and swollen mucosa, but no radiographic bone loss; Group C (periimplantitis) - radiographically confirmed pathological bone loss. After this classification, all periimplant sulci were probed at 4 sites (mesial, distal, buccal, lingual/palatal). Patients' mean age was 51.7±12.4 years, 77 women and 57 men, with a total of 486 osseointegrated endosseous implants.
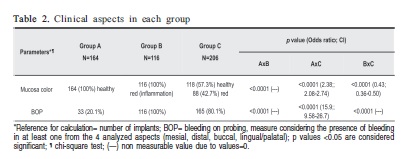
RESULTS: Groups A and C showed significant difference in age and implant region distribution (p=0.009 and p=0.008, respectively). After initial clinical and radiographic diagnosis of periimplant status, 33 (20.1%) regions showed BOP in group A. All regions in Group B presented BOP. In Group C, 41 (19.9%) regions showed no BOP. All groups differed significantly considering BOP as diagnosis parameter (p<0.0001).
CONCLUSIONS: BOP was always present in inflamed mucosa, but it was not always absent in healthy mucosa. Not all periimplantitis regions showed BOP. Clinical and radiographic aspects must always be considered together for diagnosis of PID, even if BOP is absent.
Keywords: inflammation, periimplantitis, diagnosis.
Introduction
The soft and hard tissues around endosseous implants share some similarities with the periodontium. However, differences such as the absence of cementum and periodontal ligament in the periimplant region, orientation of the collagen fibers in the periimplant soft tissue, which is parallel to the implant surface and not inserted in the implant surface and periimplant vascularization must be taken into consideration to provide reliable prognosis1.
In natural dentition, the junctional epithelium provides sealing on the baseof the periodontal sulcus against the penetration of chemical pathogens and bacterial substances2. Rupture of this sealing or lysis of connective tissue fibers attached to the apical cementum to the junctional epithelium, lead to rapid migration of the sulcular epithelium and consequent pathological pocket formation. Since cementum or fiber attachment is not seen around the titanium surface, mucosal seal provides the main barrier against the dissemination of pathological aggressions in the deep periimplant tissues. The sealing around endosseous implants, which has weak adherence to the titanium structure, is provided by the presence of junctional epithelium, sulcular epithelium and connective tissue by hemidesmosomes. The destruction of the mucosal integrity around the titanium leads to the direct extension of the pathological pocket to the bone tissue, which may result in loss of the endosseous implant1-3.
Several reports emphasize the importance of the presence of healthy gingival tissues around dental implants as being the key factor not only for aesthetics, but also for long-term success2,4-5. However, correct and early clinical diagnosis of periimplant disease status is frequently critical, which makes maintenance of the periimplant tissue difficult6. According to the Seventh European Workshop on Periodontology, the clinical parameters that indicate periimplant disease are bleeding on probing (BOP) and increased probing depth7. Clinical studies have shown that the key parameter for the diagnosis of periimplant mucositis is bleeding on gentle probing. Periimplantitis is characterized by changes in the level of the crestal bone in conjunction with BOP with or without concomitant deepening of periimplant pockets. Presence of pus, gingival recession, fistula, edema and hyperplasia are other common conditions found in periimplantitis sites6,8. However, the radiographic detection of periimplant bone loss shows only the involvement of the proximal areas to the implant, and thus periimplant probing as a diagnostic procedure is advisable to detect bone loss on all faces9. In addition, probing in periimplant sulci allows evaluating the clinical probing depth, the distance between the marginal soft tissue and a reference point on the implant (for identification of hyperplasia or gingival recession), BOP and suppuration from the periimplant pocket10.
Regarding the clinical probing depth, it is important to consider that in inflamed tissues around the implants the probe penetrates close to the bone level, while in healthy tissues the probe tip tends to stop at the histological level of connective tissue attached to the implant. The inflamed tissue with loss of connective tissue does not seem to inhibit the penetration of the probe beyond the apical extension of the junctional epithelium11-12. Quirynem et al.13 (1991) found a relation between the bone level identified by the radiographic exam and the penetration of the probe into the periimplant tissue. In screw-retained implants, the probe tip stops 1.4 mm coronally to the bone level.
This way, despite the fact that BOP is a diagnosis for periimplant disease, it is important to mention that, according to Ericsson and Lindhe14 (1993), bleeding, though unusual in healthy periodontium, is frequently found in most healthy periimplant tissues. Ferreira et al.15 (2006) stated that it is still not clearly defined if BOP of periimplant tissues would be a parameter for identifying the presence of periimplant disease. Some studies suggest that periimplant mucosa may be more sensitive to probing forces, causing more BOP when compared with teeth16-17.
The correct diagnosis of periimplant disease is a critical procedure, which makes it difficult the periimplant tissue maintenance6. Actually, a clinical standard to diagnose periimplant disease is based on the presence of BOP with probing pocket depth e"4 mm for mucositis diagnosis and additional radiographic bone loss for correct periimplantitis diagnosis8. During the first year after abutment connection, 1 mm of marginal bone loss is allowed, followed by 0.2 mm loss per year18. Currently, these criteria are still frequently referred to as the "gold standard" for implant success19.
In the present study, we considered previously established clinical characteristics of periimplant tissues that justify the exclusion criterion of BOP to diagnose the presence of periimplant disease. Based on periimplant anatomy, the tested hypothesis is that healthy periimplant tissues can also present BOP. Thus, the aim of this study was to assess if BOP is directly related to the presence of periimplant disease.
Material and methods
Clinical study procedures were conducted according to the Veiga de Almeida University Ethical Board's recommendations (Process# 238/10).
Patient Selection
One hundred and thirty-four nonsmoking patients without any systemic disease (77 women and 57 men; mean age of 51.7±12.4 years), presenting a total of 486 osseointegrated endosseous implants, 295 in the maxilla and 191 in the mandible, were randomly selected for this study (Table 1). Patients signed an informed consent form after receiving full information about the study nature and purposes.
Patients were admitted to the study if they had no medical complications, were not taking medications affecting periodontal status as described by Soskolne20 (1997) and had immediate postoperative radiographs showing the vertical bone level around the implant in order to compare bone levels after osseointegration period. Patients who had undergone any periodontal or periimplant therapy within the last six months were excluded from the study.
All periimplant regions were clinically and radiographically evaluated. Clinical examination of the periimplant sites consisted of visual inspection and palpation, analysis of mucosa color, plaque accumulation, edema and implant mobility. Conventional periapical radiographs using the paralleling technique measured the presence of vertical bone loss adjacent to the implants. The height of periimplant bone around the implant was recorded according to the exposure of the screw. According to the clinical and radiographic characteristics of the periimplant sites, patients were divided into 3 groups. Patients in Group A (healthy sites) showed no visual clinical signs of inflammation in the periimplant mucosa and no signs of bone loss. In Group B, periimplant sites characterized as mucositis, presence of mucosae presenting red color and swelling, but no signs of pathologic bone loss. Patients in Group C (periimplantitis sites) showed implant mobility and suppuration in some cases, and radiographic signs of pathologic bone loss (more than 2 screws exposed).
After initial classification, the periimplant sulci from Groups A, B and C were gently probed at 4 sites around each implant and the presence of BOP was recorded by a previously trained clinician. Periimplant measurements were recorded using a millimeter conventional U.N.C. periodontal probe, (Hu-Friedy™, Chicago, IL, USA). Then, if bleeding was detected at any of the sites, a classification of BOP was established.
Statistical Analysis
The data of each, including clinical and radiographic characteristics, were submitted to descriptive statistical analyses considering age, gender, region and presence of BOP using the statistical software SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). Numerical variables were expressed as frequencies and percentages. The chi-square test was performed to assess the significance of nominal variables between groups. Continuous variables as age were expressed as mean and standard deviation. Then, after Shapiro-Wilk test, ANOVA was applied and parametric analysis (Student's t-test) was used to compare means between groups considering that the variable had a normal distribution. The significance level was set at 5%.
Results
Taking into consideration baseline characteristics, Groups A and C showed statistically significant difference in age and implant region distribution (p=0.009 and p=0.008, respectively). In Group A (healthy periimplant tissue), 131 (19.1%) periimplant regions were characterized by the absence of BOP while 33 (20.1%) regions showed BOP with no clinical or radiographic signs of inflammation. All periimplant regions (100%) in Group B (periimplant mucositis) characterized by clinical signs of inflammation (red color of mucosa and swelling) and no radiographic bone loss, presented BOP. In Group C (periimplantitis), 165 (80.1%) regions around the implants showed BOP together with pathologic bone loss and 41 (19.9%) regions presented no signs of BOP even with bone loss. Periimplant mobility was present in 6 implants in Group C (2.9%). Group A showed no inflammation in mucosa while group B showed inflammation in all periimplant mucosal tissues, helping differential clinical diagnosis. However, group C showed 118 (57.3%) regions without any sign of mucosal inflammation even when pathological bone loss was present. These results distinguished one group from another by this criterion (p<0.0001). All groups had significant differences considering BOP (p<0.0001). Periimplant disease groups (B and C) showed higher incidence of BOP compared to group A, which showed lower incidence of BOP, despite the presence of bleeding. In addition, when comparing disease groups, higher incidence of BOP was observed in group B (p<0.0001 in all analyses comparing BOP among the 3 groups). Table 2 shows the clinical and radiographic findings in each group.
Discussion
This study evaluated the presence of BOP in periimplant regions clinically and radiographically characterized as healthy, mucositis and periimplantitis, excluding BOP as the initial diagnostic factor. Healthy patients presented no signs of visual clinical inflammation (red color or swelling) or radiographic bone loss. Regions affected by mucositis were characterized by visible clinical mucosal inflammation without signs of bone loss, and periimplantitis regions (Group C) were characterized as all regions with bone loss and more than two exposed screws, considering the radiography obtained to determine alveolar bone levels after physiologic remodeling. The main question was: The presence of BOP is really reliable when used as the unique parameter for disease diagnosis? This study showed that after the initial diagnosis considering other clinical and radiographic parameters of periimplant disease, the presence of BOP was secondary for disease identification, taking into consideration that healthy periimplant mucosae (without inflammation or bone loss) showed BOP in 20% of cases.
According to the Seventh Workshop of Periodontology7 (2011) the presence of BOP characterizes periimplant disease. However, despite BOP being a diagnosis of periimplant disease, bleeding, unusual in healthy periodontium, is found in most healthy periimplant tissues14 as evident in this work. Therefore, according to Ferreira et al.15 (2006) it has not been clearly defined whether periimplant BOP could represent a reliable parameter for identifying the presence of periimplant disease. Some studies suggest that periimplant mucosa may be more sensitive to probing forces, causing more BOP when compared with teeth16-17. Luterbacher et al.21 affirm that absence of BOP represents a stable periimplant condition. However, lack of keratinized tissue, a common finding after implant placement surgery, could also simulate an inflamed tissue, due to gingival manipulation and its red aspect, which can also be associated with non-keratinized mucosa. Therefore, swelling, pus and radiographic findings were considered for diagnosis of mucositis.
The present study showed that 20% of patients considered clinically and radiographically healthy had BOP and all the periimplant regions with a clinical aspect of inflammation (Group B - mucositis) had BOP, which lead to the conclusion that BOP is always present in inflamed mucosa, but it will not always be absent in healthy mucosa, obviously due to periimplant anatomical reasons that, even in healthy conditions, do not limit penetration of the probe beyond the barrier in the epithelial junction. However, future studies are required, including in vivo analysis in order to show how the probe penetration can stimulate bleeding in healthy and diseased periimplant mucosa.
Quirynem et al.13 (1991) found a relation between the bone level identified by the radiographic exam and the penetration of the probe into the periimplant tissue. In screwretained implants, the probe tip appears to stop 1.4 mm coronally from the bone level. In addition, the type of probe used to measure clinically the depth does not seem to influence the result. Christensen et al.22 used different types of probe to characterize CPD around endosseous implants and they concluded that the differences between the analyzed probe types during the research were not larger than 0.1 mm. In this study only one type of probe was used, which standardized the obtained results.
Another important consideration is that previous studies claim that when changes in the clinical parameters indicate disease (BOP, increased probing depth); the clinician is encouraged to take a radiograph to evaluate possible bone loss. The results of the performed research showed that 56% of the regions affected by pathological bone loss (periimplantitis – Group C) showed healthy mucosa, which in many cases leads the clinician not to perform a radiographic exam and to an erroneous healthy diagnosis. Therefore, the radiographic exam must be always considered as a followup measure and not only due to the presence of BOP, for if BOP is not present and bone loss has been triggered, possible subclinical periimplantitis may be developing. The clinical aspect as well as the radiographic aspect must be used as a diagnostic factor of periimplant disease, even if BOP is absent, instead of BOP guiding the radiographic analysis. In Implantology, the follow-up should be performed by clinical and radiographic examination at least once a year, to identify underlying bone loss in an apparently healthy periimplant gingival tissue and restore bone health before the implant failure. In case of rapid progression of periimplantitis, the following question arises: would rapid progression of periimplantitis be a consequence of a late diagnosis based solely on the clinical aspect of the mucosa?
From all patients with more than two exposed threads (pathological bone loss), 20% did not show BOP. How can this be explained? The study hypothesis is that in some patients, even with pathological bone loss, the mucosal epithelium remains adhered limiting the penetration of the probe into the tissue due to some of the following reasons: (1) bacterial penetration into the connective tissue is faster and triggers a more aggressive inflammatory response in periimplant bone, which would justify progressive bone loss without prior involvement of the mucosa; (2) at some point, mucosal inflammation might occurr with subsequent periimplant bone loss and spontaneous mucosal healing after routine cleaning procedures performed by the patient, as mucositis is characterized for being a reversible lesion, but the underlying bone shows pathological loss resulting from prior involvement due to the irreversibility of periimplantitis; (3) the thickness of the mucosa may influence the dissemination of the disease to the underlying bone limiting the damage to the thick mucosa, but further studies are needed.
Correct diagnosis of periimplant disease is still difficult to establish. Mobility of implants indicates the final stage of the disease, characterized by complete loss of the bone/implant interface10. Therefore, according to Heitz-Mayfield6 (2008), mobility cannot be a parameter for early diagnosis of periimplant disease, but it may indicate complete lack of osseointegration, which requires the implant to be immediately removed. In order to be able to intervene in the development of periimplantitis before advanced bone loss, it is important to diagnose the disease in its initial stage10. In addition, according to Leitão et al.23 (2005) even when significant inflammatory signs are absent in periimplant tissue, the qualitative detection of pathogens may indicate risk of periimplantitis, requiring stricter postoperative control.
In summary, several cases of failure found in this research were not directly related to the presence of BOP. BOP is always present in inflamed mucosa, but it will not always be absent in healthy mucosa. It is also important to consider that not all tissues presenting pathologic bone loss show clinical signs of inflammation. In the present study was considered that BOP alone cannot distinguish between the presence and absence of periimplant health and other factors involving a thorough clinical and radiographic characterization of the disease should be considered. The clinical aspect as well as the radiographic aspect must be always used as a diagnostic factor of periimplant disease, even if BOP is absent. The authors expect that this research can contribute to a better diagnosis of periimplant disease, thus reducing the rate of failures in implantology.
Acknowledgements
The authors would like to CAPES/FAPERJ for granting funds for this study (E-26/102.288/2010).
References
1. Jovanovic SA. Diagnosis and treatment of peri-implant diseases. Curr Opin Periodontol. 1994; 194-204. [ Links ]
2. Sumi T, Braian M, Shimada A, Shibata N, Takeshita K, Vandeweghe S et al. Characteristics of implant CAD/CAM abutment connections of two different internal connection systems. J Oral Rehabil. 2012; 39: 391-8. [ Links ]
3. Santos MCLG, Campos MIG, Line SRP. Early dental implant failure: a review of literature. Braz J Oral Sci. 2002; 1: 103-11. [ Links ]
4. Kan JY, Rungcharassaeng K, Lozada JL. Bilaminar subepithelial connective tissue grafts for immediate implant placement and provisionalization in the esthetic zone. J Can Dent Assoc. 2005; 33: 865-71. [ Links ]
5. Pelegrini AA, Costa CES, Sendyk WR. Connective tissue graft: a clinical alternative perimplant aesthetics. Case report. Implant News. 2006; 3: 249-54. [ Links ]
6. Heitz-Mayfield LJA. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol. 2008; 35: 292-304. [ Links ]
7. Lang NP, Berglundh T. On Behalf of Working Group 4 of the Seventh European Workshop on Periodontology: Periimplant diseases: where are we now? – Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011; 38(Suppl. 11): 178-81
8. Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008; 35: 286-91. [ Links ]
9. Albrektsson TO, Isidor F. Consensus report of session IV, 1994 apud Salvi GE, Persson GR, Heitz-Mayfield LS, Frei M, Lang NP. Adjunctive local antibiotic therapy in the treatment of peri-implantitis II: clinical and radiographic outcomes. Clin Oral Implants Res. 2007; 18: 281-5. [ Links ]
10. Mombelli A, Muhle T, Bragger U, Lang NP, Burgin WB. Comparison of periodontal and peri-implant probing by depth-force pattern analysis. Clin Oral Implants Res. 1997; 8: 448-54. [ Links ]
11. Lang NP, Wetzel AC, Stich H, Caffesse RJ. Histologic probe penetration in healthy and inflamed periimplant tissues. Clin Oral Implants Res. 1994; 5: 191-201. [ Links ]
12. Khammissa RA, Feller L, Meyerov R, Lemmer J. Peri-implant mucositis and peri-implantitis: clinical and histopathological characteristics and treatment. SADJ. 2012; 67: 124-6. [ Links ]
13. Quirynen M, Van Steenberghe D, Jacobs R, Schotte A, Darius B. The reliability of pocket probing around screw type implants. Clin Oral Implants Res. 1991; 2: 186-92. [ Links ]
14. Ericsson L, Lindhe J. Probing depth at implants and teeth. An experimental study in the dog. J Clin Periodontol. 1993; 9: 623-7. [ Links ]
15. Ferreira SD, Silva GL, Cortelli JR, Costa JE, Costa FO. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J Clin Periodontol. 2006; 33: 929-35. [ Links ]
16. Casado PL, Otazu IB, Balduino A, Mello W, Barboza EP, Duarte MEL. Identification of periodontal pathogens in healthy periimplant sites. Implant Dent. 2011; 20: 226-35. [ Links ]
17. Lopez-Piriz R, Morales A, Giménez MJ, Bowen A, Carroquino R, Aguilar L et al. Correlation between clinical parameters characterising peri-implant and periodontal health: A practice-based research in Spain in a series of patients with implants installed 4-5 years ago. Med Oral Patol Oral Cir Bucal. 2012; 17: e893-901. [ Links ]
18. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986; 1: 11-25. [ Links ]
19. Levin L. Dealing with dental implant failures. J Appl Oral Sci. 2008; 16: 171-5. [ Links ]
20. Soskolne WA. Subgingival delivery of therapeutic agents in the treatment of peridontal diseases. Critic Rev Oral Biol Med. 1997; 8: 164-74. [ Links ]
21. Luterbacher S, Mayfield I, Bragger U, Lang NP. Diagnostic characteristics of clinical and microbiological tests for monitoring periodontal and periimplant mucosal tissue conditions. Clin Oral Implan Res. 2000; 11: 521-9. [ Links ]
22. Christensen MM, Joss A, Lang NP. Reproducibility of automated periodontal probing around teeth and osseointegrated oral implants. Clin Oral Implant Res. 1997; 8: 455-64. [ Links ]
23. Leitão JA, De Lorenzo JL, Avila-Campos MJ, Sendyk WR. Analysis of the presence of pathogens which predict the risk of disease at peri-implant sites through polymerases chain reaction. Braz Oral Res. 2005; 19: 52-7. [ Links ]
 Correspondence:
Correspondence:
Priscila Ladeira Casado
Núcleo de Terapia Celular
Unidade de Pesquisa Clinica
Rua Marques de Paraná 303, 40° andar,
CEP: 24033-900 - Centro, Niterói, RJ, Brasil
E-mail: pcasado@into.saude.gov.br
Received for publication: February 21, 2013
Accepted: June 11, 2013













