Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.12 no.2 Piracicaba Abr./Jun. 2013
ORIGINAL ARTICLE
Oral antibacterial effect of chlorhexidine treatments and professional prophylaxis in children
Thiago Cruvinel SilvaI; Thaís Marchini Oliveira ValarelliI; Vivien Thiemy SakaiII; Vanessa TessarolliII; Maria Aparecida de Andrade Moreira MachadoI
IDepartment of Pediatric Dentistry, Orthodontics, and Public Health, Bauru School of Dentistry, University of São Paulo, Bauru, SP, Brazil
IIDepartment of Clinic and Surgery, Alfenas, School of Dentistry, Alfenas Federal University, MG, Brazil
ABSTRACT
AIM: The primary aim of this longitudinal study was to evaluate additional effects of 4-week chlorhexidine digluconate (CHX) gel treatments to control Aggregatibacter actinomycetemcomitans counts in children after professional dental prophylaxis. Porphyromonas gingivalis and Streptococcus mutans counts were also determined to evaluate the secondary effects of anti-plaque treatments on microbial shifts.
METHODS: Twenty-six children with A. actinomycetemcomitans counts >4 log10/mL of saliva and/or Quigley-Hein plaque index >3.0 were enrolled in this study. Patients were randomly assigned to groups GI (placebo gel), GII (0.5% CHX gel), GIII (1% CHX gel), and GIV (2% CHX gel). Four sessions of treatment were performed during 4 weeks after a session of professional dental prophylaxis. Real-Time polymerase chain reaction (PCR) was used to determine viable microorganism counts in non-stimulated whole saliva samples collected at baseline, one week, one month and three months after interruption of treatments.
RESULTS:A reduction of all bacterial counts was detected after the 3-month follow-up in all groups. Lower counts of P. gingivalis were achieved from 1 week on after treatments. The 2% CHX concentration seemed to contribute to lower A. actinomycetemcomitans levels and increase S. mutans levels.
CONCLUSIONS: Professional dental prophylaxis was effective to control salivary levels of A. actinomycetemcomitans, P. gingivalis and S. mutans. Additional antimicrobial effects, however, were not observed by the combination of professional dental prophylaxis and 4-week chlorhexidine gel treatments.
Keywords: aggressive periodontitis, professional prophylaxis, chlorhexidine, Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Streptococcus mutans.
Introduction
Aggressive periodontitis (AgP) affects younger individuals and is characterized by a rapid loss of the supporting connective tissue and alveolar bone around teeth in the presence of oral biofilm1. The prevalence of AgP is higher among Black individuals than among Caucasian individuals, ranging from 0.1% to 7.6%, with a tendency for familiar clustering of cases2.
Increased detection levels of Aggregatibacter actinomycetemcomitans in the oral cavity is an indicator for the risk of development of AgP in children, adolescents and young adults3-6. A. actinomycetemcomitans is a Gram-negative facultative anaerobic microorganism acquired via maternal transmission7-8. It plays an important role in the onset of localized AgP because of its virulence factors, such as RTX leukotoxin and cytolethal distending toxin, which triggerimmunological host responses activating osteoclastogenesis and leading to tissue breakdown9.
Antimicrobial chemotherapeutic methods can be used to prevent AgP. Chlorhexidine digluconate (CHX) is effective against a large spectrum of Gram-positive and Gram-negative oral microorganisms10. Short-term adjunctive use of CHX gel and/or mouthrinses decreases periodontopathogen counts11-12 and limits supra/subgingival biofilm re-growth after mechanical removal of dental plaque13-16. Microbial recolonization of tooth surfaces, however, begins soon after antimicrobial treatments17, which might induce shifts in the composition of biofilms. Simultaneous identification of certain bacteria may also change the clinical severity of periodontal diseases. Ramsey and Whiteley18 (2009) demonstrated an enhanced resistance and virulence of A. actinomycetemcomitans in the presence of oral streptococci. De Soete et al.19 (2005) observed higher Streptococcus mutans counts in saliva of periodontal patients after scaling root planning (SRP). Mayorga-Fayad et al.20 (2007) reported more prevalent detection of Porphyromonas gingivalis in AgP patients.
To the best of our knowledge, salivary microbial shifts after the association between mechanical removal of dental plaque and CHX gel treatments have not yet been investigated. The primary aim of this longitudinal study was to evaluate additional effects of 4-week CHX gel treatments to control A. actinomycetemcomitans counts in children after professional dental prophylaxis. P. gingivalis and S. mutans counts were also determined to evaluate the secondary effects of anti-plaque treatments on microbial shifts.
Material and methods
Participants
The present study was conducted after approval by the Ethics Committee of Bauru School of Dentistry, University of São Paulo (Process #47/2006). Parents of patients attending the Clinic of Pediatric Dentistry were invited to enroll their children in the study. Parental agreement was documented by signing a written informed consent.
In a preliminary screening, forty-eight 7-12-year-old children donated saliva and were examined by a calibrated dentist (intraobserver concordance 8>0.9) to determine Löe and Silness21 (1963) gingival index (G-index) and Quigley & Hein modified by Turesky dental plaque index22 (DPindex). Patients with A. actinomycetemcomitans counts >4 log10/mL of saliva and/or DP-index >3.0 could be included in the study. Consumption of antibiotics and non-steroidal anti-inflammatory drugs up to 30 days before the study, presence of supragingival calculus, severe malocclusion, oral lesions in soft tissues and use of orthodontic appliances were considered as exclusion criteria.
Twenty-six children were enrolled in the study and randomly assigned to groups GI (control, placebo gel), GII (0.5% chlorhexidine gel), GIII (1% chlorhexidine gel), and GIV (2% chlorhexidine gel) by Microsoft Excel 2003® software.
Clinical procedures
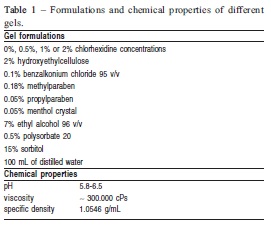
Non-stimulated whole saliva was collected into sterile glass beaker for 5 min. Saliva samples were stored at -20oC until DNA extraction of specific microorganisms23. Professional dental prophylaxis with sodium bicarbonate jet was performed before the first session of treatment. For that purpose, dental plaque was previously disclosed by a solution of 0.2% fuchsine to ensure the quality of procedures. Then, CHX gels were dispensed into disposable trays and applied on teeth for 1 min. Formulations and chemical properties of CHX gels are depicted in Table 1. Excesses of gel were removed and children were instructed to restrict liquid and food intake for next 30 min. Treatments were conducted once a week for 28 days. Clinical appointments were scheduled 1 week, 1 month and 3 months after the interruption of gel treatments to collect saliva samples and determine gingival and dental plaque indexes.
Real Time PCR tests
Real time PCR tests were performed to allow the detection and quantification of A. actinomycetemcomitans, P. gingivalis, and S. mutans. After defrosting and homogenizing the saliva samples, two aliquots of 500 μL saliva each were diluted in 1 mL of sterile demineralized water (1:2) and centrifuged (10,000 xg) at 4oC for 5 min. Supernatants were discarded and pellets were washed three times with sterile demineralized water (10000 xg, 4oC, 5 min)23. One bacterial pellet was incubated with 100 μL of a solution for cellular lyses (20 U/mL mutanolysin plus 0.2 mg/mL lysozyme) at 37oC for 2 h. This mixture was boiled (100oC, 10 min) to extract bacterial DNA from S. mutans cells. Other bacterial pellet was used to extract DNA from A. actinomycetemcomitans and P. gingivalis cells. The pellet was suspended in a mixture of 100 μL DNAse-free water and 100 μL InstaGene Matrix (Bio-Rad Laboratories Inc., Hercules, CA, USA), and incubated at 56oC for 30 min. Then, the samples were vortexed and boiled for 10 min. Unbroken cells and large debris were removed by subsequent centrifugation(10,000 xg, 3 min). Supernatants were used for Real-time PCR DNA analysis.
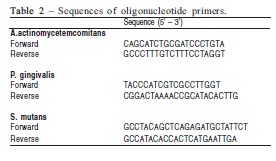
Real-Time PCR DNA analysis was performed in an ABI Prism 7000 system (Applied Biosystems, Warrington, UK), 1X Universal PCR Master-Mix (ABI), 200 nM of specific primers, 1μL of the supernatant, and 250 nM of each quencher dye (6-carboxyfluorescein and 6-carboxytetramethylrhodamine). The specificities of the primers were initially confirmed by BLAST with the National Center for Biotechnology Information server (http://www.ncbi.nlm.nih.gov). Oligonucleotide primer sequences are shown in Table 2. Bacterial DNA levels were determined using the Ct method and normalized by the volume of the supernatants.
Statistical analysis
Data were analyzed by NCSS/PASS® 2000 statistical software (Kaysville, UT, USA). Microorganism counts were log-transformed before statistical tests. Normality and homogeneity of data were respectively evaluated by Kolmogorov-Smirnov and Levene tests. Statistical differences were assessed using parametric one-way ANOVA and Bonferroni post-hoc test. Correlations between gingival or dental plaque indexes and microorganism counts were observed by Pearson's test. The p values <0.05 were considered significant.
Results
Gingival and Dental Plaque Indexes
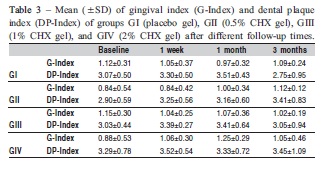
Fourteen male and 12 female children were enrolled in the study (8.9±1.2 years). Correlations between gingival and dental plaque indexes and A. actinomycetemcomitans (GIndex r=-0.24, DP-Index r=-0.10), P. gingivalis (G-Index r=-0.03, DP-Index r=-0.10), and S. mutans (G-Index r= -0.14, DP-Index r=0.01) counts were not observed at baseline. Both clinical indexes were statistically similar in different treatment groups and times (Table 3).
Microorganism counts
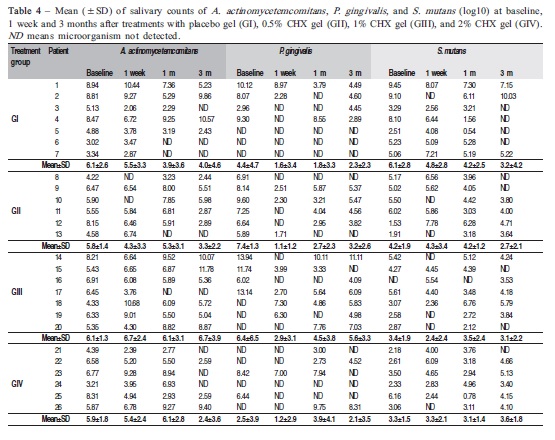
P. gingivalis and S. mutans were identified in 61.5% and 96.2% of children, respectively, at baseline. Bacterial counts did not differ significantly between groups throughout the study (p>0.05). The effectiveness of treatments was demonstrated by statistical differences observed among bacterial counts in different follow-up times. Considering all groups (Table 4), the mean of A. actinomycetemcomitans counts at 3 months (4.20±3.90) was significantly lower than at baseline (5.98±1.96) (p<0.05). Similar differences were verified in S. mutans counts between 3-month follow-up (3.14±2.67) and baseline (4.29±2.31) (p<0.05). P. gingivalis counts were statistically reduced from 1 week (1.73±2.74) after interruption of treatments compared with baseline (5.18±4.72) (p<0.05). Although P. gingivalis counts gradually increased to 3.21±3.42 at 1-month and 3.37±3.12 at 3-month follow-ups, the levels still remained significantly lower than at baseline (p<0.05).
The maintenance of lower P. gingivalis and S. mutans counts were noticed in 100% and 84.6% children, respectively, with initial P. gingivalis and S. mutans counts >4 log10/mL of saliva. On the other hand, respectively 54.5% and 69.2% children with initial P. gingivalis and S. mutans counts <4 log10/mL of saliva showed higher microorganism counts after 3 months of follow-up (Figures 1A, B and C).
A reduction in the percentage of children with salivary A. actinomycetemcomitans counts >4 log10/mL of saliva was observed in all groups since CHX treatments. The highest CHX concentration (2%) seemed to contribute to obtain lower levels of A. actinomycetemcomitans in most patients at the end of the study (Figure 1A).
In comparison with baseline, the percentage of patients with P. gingivalis counts >4 log10/mL of saliva decreased after 1 week and 1 month of placebo and 0.5% CHX gel treatments, whereas the percentage of patients did not vary after 1% and 2% CHX gel treatments. After 3 months, different CHX concentrations differed in controlling P. gingivalis levels. Higher percentage of children with P. gingivalis counts >4 log10 was noticed in the group GIII as compared to groups GII and GIV (Figure 1B).
The results supported a trend of simultaneous increment of CHX gel concentrations and S. mutans counts along time. Treatments with 2% CHX gel increased S. mutans levels in most patients 1 week and 3 months after CHX treatments (Figure 1C).
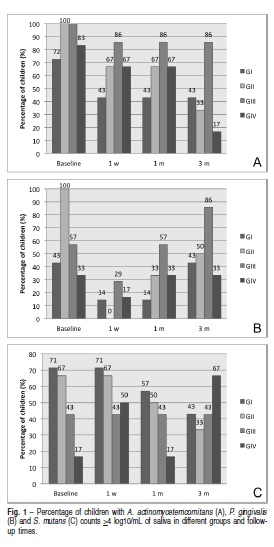
Discussion
A positive impact on the prevention of AgP could be achieved by a short-term reduction of salivary periodontopathogens. This preliminary study demonstrated that one session of professional dental prophylaxis can be effective to reduce salivary counts of A. actinomycetemcomitans, P. gingivalis and S. mutans in children. Additional antimicrobial effects of CHX gels could not be demonstrated. After a 3- month follow-up, most children showed lower salivary counts of at least one microorganism, especially in patients with specific bacterial counts >4 log10 at baseline.
The amounts of microbial deposits are inconsistent with the severity of periodontal tissue destruction in AgP patients23. High proportions of A. actinomycetemcomitans and P. gingivalis can be associated with the onset of this disease. The rapid identification and quantification of periodontal microorganisms, therefore, permits early treatments to prevent the colonization of tooth surfaces for the maintenance of homeostasis of the periodontium. Currently, it is possible to monitor oral microbial compositions using diagnostic tools as Real-Time PCR24-25. Umeda et al.23 (1998) demonstrated that PCR is a specific and highly sensitive method for detection of periodontopathogens in whole saliva. Our study focused on the potential application of CHX gels to reduce salivary pathogens, which would be able to colonize tooth and radicular surfaces, and increase the risk of developing periodontal diseases.
In the present study, disposable trays were used to deliver CHX gels in the oral cavity of children. Dental plaque and gingival indexes of all groups did not differ significantly after treatments. Francis et al.14 (1987) demonstrated efficient inhibition of dental plaque and control of gingival bleeding in patients treated with 4-week tray application of 1% CHX gel. Slot et al.16 (2010) reported an improvement of dental plaque control after tray application of 1% CHX gel compared to 0.12% CHX dentifrice gel. Our contrasting results could be explained by the inclusion of healthy periodontal patients with low scores of dental plaque and gingival indexes at baseline. According to Berchier et al.26 (2010), the increase of CHX concentration seems to enhance the dental plaque control, although this could not be considered clinically relevant.
Since the diversity of microorganisms from saliva and dental plaque has been estimated in about 19,000 specieslevel phylotypes27, multiple microbial clusters are possible among individuals. In our study, the host-specific microbial load can be illustrated by the detection of P. gingivalis and S. mutans in respectively 61.5% and 73% children at baseline. Similar treatments resulted in diverse antimicrobial responses in different patients: 4-week 1% CHX gel treatment was effective to reduce all bacterial counts in patient #17, whereas the same regimen increased all microorganism counts in patient #18. Lower S. mutans and P. gingivalis counts and higher A. actinomycetemcomitans were detected in patients #14 and #15. Filoche et al.28 (2008) showed a host specificity of antimicrobial agents against ex vivo microorganisms.
After a 3-month follow-up, professional dental prophylaxis was effective to reduce at least one specific bacterial count in all patients of group GI. Periodic dental visits may also have contributed to the enhancement of dental plaque control in children. The application of 0.5% CHX gel decreased simultaneously A. actinomycetemcomitans, P. gingivalis and S. mutans counts in most patients. Higher counts of bacteria were detected in four out of seven patients treated with 1% CHX gel. Two percent CHX gel treatments were responsible for lowering A. actinomycetemcomitans counts in 83.3% patients and raising S. mutans counts in 66.7% patients at the end of the study. Although speculative, our findings could indicate a possible trend of microbial shifts between periodontopathogens and cariogenic microorganisms with increasing concentration of CHX gels. De Soete et al.19 (2005) reported a significant increase of detection of S. mutans after SRP. Clinical interventions could induce local shifts in composition of biofilms from predominant anaerobic and proteolytic microorganisms to aerobic and acidogenic microorganisms. Other microbial relationships can be expressed by association among specific substrate, microbiological composition and metabolism. For example, lactate - a metabolite of cariogenic bacteria - can be used as a carbon source for Veilonella sp. These bacteria are able to produce considerable amount of menaquidone, an organic source of vitamin K, essential to growth of P. gingivalis and T. denticola29.
Thresholds of minimal P. gingivalis and S. mutans counts were not established in our inclusion criteria. Independently of treatment group, most patients with initial S. mutans and P. gingivalis counts >4 log10/mL of saliva presented a trend to reduction of bacterial counts after 3-month follow-up, whereas respectively 69.2% and 54.5% patients with S. mutans and P. gingivalis counts <4 log10/mL of saliva presented a trend to increase bacterial counts along time. Moreover, faster reductions of P. gingivalis counts seemed to indicate more sensitivity to mechanical and chemotherapeutic treatments than A. actinomycetemcomitans and S. mutans. These findings suggest shifts in microbial compositions after treatments for dental plaque control. Sekino et al.12 (2004) demonstrated that the combination of a 0.2% CHX mouthrinse and a 1% CHX gel decreased salivary microorganisms and blocked tooth recolonization by A. actinomycetemcomitans and P. gingivalis. A significant reduction of periodontopathogen counts after SRP and fullmouth disinfection with CHX was also described by Quirynen et al.17 (1999). Perinetti et al.11 (2004) achieved a reduction of A. actinomycetemcomitans and S. mutans counts in periodontal pockets after irrigation with 1% CHX gel.
Some issues should be discussed regarding the results. It is possible that significant differences were not observed among the different groups of this study due to some limitations: (1) small sample size, (2) allocation of participants into four distinct groups, and (3) diversity of antimicrobial responses from each patient. Also, inclusion criteria limited the enrollment of participants in this study, especially A. actinomycetemcomitans counts >4 log10/mL of saliva. Additionally, the follow-up could be extended to 6 months to adequately assess the effect of chlorhexidine substantivity on clinical and microbiological parameters.
In conclusion, this study demonstrated that mechanical dental plaque control can be used as an effective preventive strategy to control salivary counts of A. actinomycetemcomitans, P. gingivalis and S. mutans, contributing to the maintenance of periodontal health. Further randomized clinical trials are needed to establish safe protocols for demonstrating additional benefits of chlorhexidine treatments against pathogenic microorganisms in saliva, with minimum modifications of oral homeostasis.
Acknowledgments
The authors thank Thiago José Dionísio for his valuable technical support. This study was financially supported by The São Paulo State Research Foundation – FAPESP, Brazil
(process # 2007/00962-6).
References
1. Albandar JM, Buischi YAP, Barbosa MFZ. Disease in adolescents. A 3-year longitudinal study. J Periodontol. 1991; 62: 370-6. [ Links ]
2. Ickenstein GW, Klotz JM, Langohr HD. Kopfschmerz bei Polycythaemia vera. Schmerz. 1999; 13: 279-82. [ Links ]
3. Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004; 34: 9-21. [ Links ]
4. Armitage GC. Comparison of the microbiological features of chronic and aggressive periodontitis. Periodontol 2000. 2010; 53: 70-88. [ Links ]
5. Jardim Jr EG, Bosco JMD, Lopes AM, Landucci LF, Jardim ECG, Carneiro SRS. Occurrence of Actinobacillus actinomycetemcomitans in patients with chronic periodontitis, aggressive periodontitis, healthy subjects and children with gingivitis in two cities of the state of São Paulo, Brazil. J Appl Oral Sci. 2006; 14: 153-6. [ Links ]
6. Shaddox LM, Huang H, Lin T, Hou W, Harrison PL, Aukhil I, et al. Microbiological characterization in children with aggressive periodontitis. J Dent Res. 2012; 91: 927-33. [ Links ]
7. Lamell CW, Griffen AL, McClellan DL, Leys EJ. Acquisition and colonization stability of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in children. J Clin Microbiol. 2000; 38: 1196-9. [ Links ]
8. Fine DH, Markowitz K, Furgang D, Velliyagounder K. Aggregatibacter actinomycetemcomitans as an early colonizer of oral tissues: epithelium as a reservoir? J Clin Microbiol. 2010; 48: 4464-73. [ Links ]
9. Henderson B, Ward JM, Ready D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: a triple A* periodontopathogen? Periodontol 2000. 2010; 54: 78-105. [ Links ]
10. Emilson CG. Susceptibility of various microorganisms to chlorhexidine. Scand J Dent Res. 1977; 85: 255-65. [ Links ]
11. Perinetti G, Paolantonio M, Cordella C, Ercole DS, Serra E, Clinical PR. Clinical and microbiological effects of subgingival administration of two active gels on persistent pockets of chronic periodontitis patients. J Clin Periodontol. 2004; 31: 273-81. [ Links ]
12. Sekino S, Ramberg P, Uzel NG, Socransky S, Lindhe J. The effect of a chlorhexidine regimen on de novo plaque formation. J Clin Periodontol. 2004; 31: 609-14. [ Links ]
13. Löe H, Schiott CR. The effect of mouthrinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodontal Res. 1970; 5: 79-83. [ Links ]
14. Francis JR, Hunter B, Addy M. A comparison of three delivery methods of chlorhexidine in handicapped children. I. Effects on plaque, gingivitis, and toothstaining. J Periodontol. 1987; 58: 451-5. [ Links ]
15. Vinholis AH, Figueiredo LC, Marcantonio Júnior E, Marcantonio RA, Salvador SL, Goissis G. Subgingival utilization of a 1% chlorhexidine collagen gel for the treatment of periodontal pockets. A clinical and microbiological study. Braz Dent J. 2001; 12: 209-13. [ Links ]
16. Slot DE, Rosema NAM, Hennequin-Hoenderdos NL, Versteeg PA, van der Velden U, van der Weijden GA. The effect of 1% chlorhexidine gel and 0.12% dentifrice gel on plaque accumulation: a 3-day non-brushing model. Int J Dent Hyg. 2010; 8: 294-300. [ Links ]
17. Quirynen M, Mongardini C, Pauwels M, Bollen CML, van Eldere J, van Steenbergh D. One stage full- versus partial-mouth disinfection in the treatment of chronic adult or generalized early-onset periodontitis. II. Longterm impact on microbial load. J Periodontol. 1999; 70: 646-56. [ Links ]
18. Ramsey MM, Whiteley M. Polymicrobial interactions stimulate resistance to host innate immunity through metabolite perception. Proc Natl Acad Sci USA. 2009; 106: 1578-83. [ Links ]
19. De Soete M, Dekeyser C, Pauwels M, Teughels W, van Steenberghe D, Quirynen M. Increase in cariogenic bacteria after initial periodontal therapy. J Dent Res. 2005; 84: 48-53. [ Links ]
20. Mayorga-fayad I, Lafaurie GI, Contreras A, Castillo DM, Barón A, Aya M del R. Microflora subgingival en periodontitis crónica y agresiva en Bogotá, Colombia: un acercamiento epidemiológico. Biomedica. 2007; 27: 21-33. [ Links ]
21. Löe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963; 21: 533-51. [ Links ]
22. Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970; 41: 41-3. [ Links ]
23. Umeda M, Contreras A, Chen C, Bakker I, Slots J. The utility of whole saliva to detect the oral presence of periodontopathic bacteria. J Periodontol. 1998; 69: 828-33. [ Links ]
24. Price RR, Viscount HB, Stanley MC, Leung KP. Targeted profiling of oral bacteria in human saliva and in vitro biofilms with quantitative real-time PCR. Biofouling. 2007; 23: 203-13. [ Links ]
25. Atieh MA. Accuracy of real-time polymerase chain reaction versus anaerobic culture in detection of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis: a meta-analysis. J Periodontol. 2008; 79: 1620-9. [ Links ]
26. Berchier CE, Slot DE, van der Weijden GA. The efficacy of 0.12% chlorhexidine mouthrinse compared with 0.2% on plaque accumulation and periodontal parameters: a systematic review. J Clin Periodontol. 2010; 37: 829-39. [ Links ]
27. Keijser BJF, Zaura E, Huse SM, van der Vossen JMBM, Schuren FHJ, Montijn RC, et al. Pyrosequencing analysis of the oral microflora of healthy adults. J Dent Res. 2008; 87: 1016-20. [ Links ]
28. Filoche SK, Soma D, van Bekkum M, Sissons CH. Plaques from different individuals yield different microbiota responses to oral-antiseptic treatment. FEMS Immunol Med Microbiol. 2008; 54: 27-36. [ Links ]
29. Hojo K, Nagaoka S, Ohshima T, Maeda N. Bacterial interactions in dental biofilm development. J Dent Res. 2009; 88: 982-90. [ Links ]
 Correspondence:
Correspondence:
Thiago Cruvinel Silva
Alameda Octávio Pinheiro Brisolla, 9-75,
Vila Universitária, CEP: 17012-901
Bauru, SP, Brasil
E-mail: thiagocruvinel@yahoo.com.br
Received for publication: March 20, 2013
Accepted: June 25, 2013













