Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.13 no.1 Piracicaba Jan./Mar. 2014
ORIGINAL ARTICLE
In Vitro antimicrobial photoinactivation with methylene blue in different microorganisms
Bruna Paloma de OliveiraI; Carla Cabral dos Santos Accioly LinsII; Fátima Alves DinizIII; Liliane Lima MeloIII; Célia Maria Machado Barbosa de CastroIV
I Universidade Federal de Pernambuco - UFPE, School of Dentistry, Department of Prosthodontics and Oral Facial Surgery, Recife, PE, Brasil
II Universidade Federal de Pernambuco - UFPE, Department of Anatomy, Recife, PE, Brasil
III Universidade Federal de Pernambuco - UFPE, Laboratory of Immunopathology Keiso Asami - LIKA, Recife, PE, Brasil
VI Universidade Federal de Pernambuco - UFPE, Department of Tropical Medicine, Recife, PE, Brasil
ABSTRACT
Aim: To evaluate the in vitro antimicrobial effects of photodynamic therapy (PDT). Methods: The microorganism indicators were: Candida albicans, Pseudomonas aeruginosa,Enterococcus faecalis and Staphylococcus aureus. A microbial pool was prepared (108 cells/mL), from which aliquots were transferred to culture plates for carrying out the PDT using methylene blue (50 μM) and low-power laser (660 nm, 100 mW and 9 J).The effect of methylene blue alone, low power laser and the absence of treatments were evaluated. Then, aliquots of 1 μL were plated in a media culture, the number of colony forming units (CFU/mL) was obtained and the data submitted to the F test (ANOVA) with Tamhane's comparisons. Results:The laser radiation in the presence of methylene blue was able to eliminate 74.90% of C. albicans, 72.41% of P. aeruginosa, 96.44% of E. faecalis and 95.42% of S. aureus, thus statistically significant differences were found among the groups (p<0.001). Conclusions: PDT was effective in reducing the number of viable cells in the studiedmicroorganisms, especially E. faecalis and S. aureus.
Keywords: endodontics; Enterococcus faecalis; methylene blue; microbiology; photodynamic therapy.
Introduction
Microorganisms play an essential role in the development and maintenance of pathologies that affect the pulp and the periapical region1, and their removal during the biomechanical preparation is crucial to the success of endodontic treatment2.
Pseudomonas aeruginosa and Staphylococcus aureus have been commonly associated with persistent infections3-5.Special attention has been given to Enterococcus faecalis, a tough Gram-positive bacterium, which has a much higher incidence in cases of endodontic treatment failure6-7. This microorganism has the property of survival in extremely alkaline pH environments, with scarce nutrients, invading and growing within dentinal tubules, colonizing the root canal and reinfecting the root-filled teeth8-9.
Fungi are occasionally found in the primary infection of root canals, but occur more frequently in teeth obturated with lesions refractory to treatment. Candida albicans is the most prevalent fungal species, a microorganism that has affinity for dentin and is resistant to some intracanal medications, for example, those based on calcium hydroxide10.
The antibacterial activity of low power lasers associated with a photosensitizer has been studied as adjuvant treatment together with conventional endodontic therapy11. Photodynamic therapy (PDT) assumes that the interaction of light with an appropriate wavelength, when associated with a nontoxic photosensitizing dye in the presence of oxygen, results in free radicals of high cytotoxicity, such as superoxides and singlet oxygen. These highly reactive species can cause serious damage to microorganisms via irreversible oxidation of cellular components12.
However, this treatment presents other challenges regarding its susceptibility to different microorganisms, according to their physiology13-14.Therefore, it is still necessary to set specific parameters so that PDT can be used for maximum effectiveness in removing microorganisms that cause endodontic infections.
The aim of this study was to contribute to other studies that seek to clarify the effects of antimicrobial PDT, evaluating the effects of in vitro photosensitization of methylene blue by laser irradiation in suspensions containing various species of microorganisms.
Material and methods
Microorganisms and preparation of microbial suspensions
The microorganisms used in the study were obtained from the Department of Microbiology and Antibiotics of the Federal University of Pernambuco, one yeast and three bacterial strains: Candida albicans (ATCC 10231), Staphylococcus aureus (ATCC 29213), Pseudomonas aeruginosa (ATCC 27853) and Enterococcus faecalis (ATCC 6057) previously cultured in Agar Nutrient (Difco, Detroit, MI, USA). Four microbial suspensions of 3 mL each were formed in test tubes, in which microorganism indicators were diluted using sterile saline (0.9% NaCl). The suspensions of the microorganisms had the optical density adjusted spectrophotometrically to approximately 1.0 x 108 colonyforming units (CFU) mL-1(equivalent to 1.0 McFarland scale)5,15. From each microbial suspension, 2 mL was removed and a mixture with the four microorganisms was prepared (microbial pool).
Description of experimental groups
Aliquots of 200μLwere removed from the microbial pool and transferred to culture plates with 24 wells each. Experimental groups were formed as follows (n=10): Group L-P-: positive control (microbial pool); Group L+P-: formed by the microbial pool that received the isolated action of the laser; Group L-P+: microbial pool that received 20μl of the photosensitizer for two minutes, and Group L+P+: microbial pool that received 20μl of the photosensitizer for two minutes and then laser irradiation.
Laser and photosensitizer
The photosensitizer used in the study was a solution of methylene blue 50μM (Chimiolux®; Hypofarma, Belo Horizonte, MG, Brazil). The light source came from a low power laser (Equipment Whitening Lase II, DMC equipment Ltd) with a wavelength of 660 nm, 100 mW, at an irradiation time of 3 min. This resulted in an energy dose of 9 J for each sample.
Photosensitization in vitro
Irradiation of samples was performed under aseptic conditions in a laminar flow hood (A/B3 CASS II; AIR TECH, Tokyo, Japan). Throughout the experiment, all the samples were handled in the dark. A bulkhead was made using an opaque black paper sheet with a central hole with a diameter similar to the wells, to prevent the same well from being irradiated more than once. A burette clamp was used in order to standardize the distance of 3 cm between the tip of the laser and the bottom of each well on the plate.
To evaluate the antimicrobial treatment, aliquots of 1μLwere obtained from each well and plated in Agar Sabouraud (Difco) growth medium and in Blood Agar (Difco). After incubation for 48 h at 37°C in a bacteriological incubator, the CFU/mL was counted through observation of the morphology of the colonies. All experiments were conducted in triplicate.
Statistical Analysis
For data analysis, statistical measures were obtained using the average and standard deviation of the colonies count (in CFU/mL). Calculation of percentage (descriptive statistics) was made using the F test (ANOVA), with comparisons using Tamhane's inferential statistics technique. The hypothesis verification of equal variances was performed using Levene's F test with p<0.001 considered as statistically significant. The statistical program used was SPSS (Statistical Package for Social Sciences) version 15 (SPSS Inc., Chicago, IL, USA).
Results
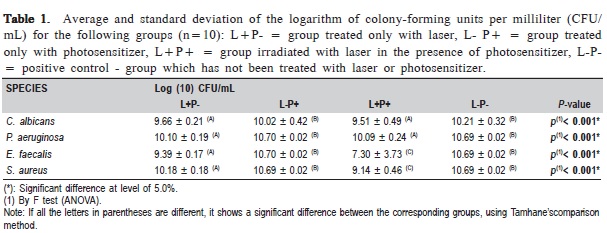
The microbial effectiveness of the group treated with laser in the presence of the photosensitizer (L+P+) in all the microorganisms tested showed the lowest average value of CFU/mLwith significant difference between the groups (p<0.001) (Table 1).
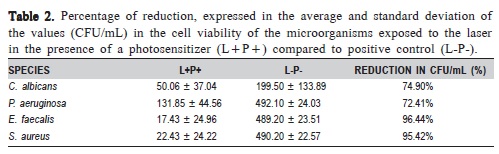
Table 2 shows the percentage of reduction in CFU/mL observed for the L+P+ group compared to the L-P-. Among the evaluated microorganisms, P. aeruginosa was the most resistant to PDT, followed by C. albicans, S. aureus and E. faecalis.
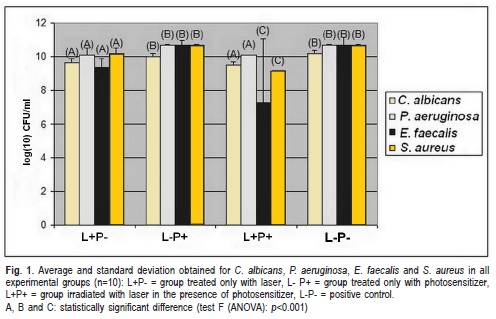
Figure 1 shows the average and the standard deviation of CFU/mL obtained for the various microorganisms studied in each experimental group. In the group L+P-, C. albicans and P. aeruginosa showed a reduction in the number of CFU/ mL, similar to L+P+; whereas in group L+P+, E. faecalis and S. aureus showed a significant reduction compared to L+P-.The CFU/mL number in L-P+ was similar to the group L-P-. When the groups L+P- andL-P+ were compared, a significant decrease of the microbial growth in all the studied microorganisms was observed.
Discussion
The application of PDT, as an adjuvant treatment, has been indicated in endodontics, seeking to help the conventional therapy in eradicating the resistant pathogens of the root canal16-19.
Microbial agents are considered the main etiological factors to the progression and perpetuation of pulp and periradicular inflammatory diseases20.The pathogens used in the present study were selected because of their clinical importance and association with endodontic infection21.
Various dyes have been used to perform PDT, such as toluidine blue and methylene blue14. The latter had its chemical properties tested in several studies that proved its antimicrobial efficacy, which motivated the choice for using this product in the present study22-24.
The results obtained in this study demonstrated that when methylene blue was used alone, there was no significant reduction in the number of CFU/mL for all studied species. This result indicates that the concentration and the amount used in the present study showed no cytotoxic effect on the test microorganisms, corroborating the findings of Pupo et al.25 (2011) and Miyabe et al.26 (2011) who used only methylene blue at 100 mg/mL in C. albicans and at 3 mM in S. aureus respectively. These results, however, are different from those of Foschi et al.27 (2007), who reported a 19.5% reduction in viability of E. faecalis when 6.25 mg/mL of methylene blue was used without photosensitization in extracted single-rooted teeth.
Regarding the laser effects in the absence of a photosensitizer, P. aeruginosa and C. albicans showed a reduction in the number of CFU/mL similar to the group treated with PDT, differing from the findings of Queiroga et al.28 (2011),who found no reduction in cell viability of C. albicans after their exposure to the laser in the parameters of 60 J/cm2, 120 J/cm2 and 180 J/cm2.
Thus, in comparison with other groups, PDT behaved better in microbial reduction using methylene blue with a concentration of 50 μM at 660 nm, 100 mW and 9J, corroborating other studies that showed that the use of the laser associated with a photosensitizer is effective against various microorganisms16,29-31.
Microbial reduction by photodynamic effect faces various challenges when used against Gram-positive bacteria, Gram-negative and fungi. E. faecalis was the microorganism with the highest reduced percentage of CFU/mL (96.44%), followed by S. aureus (95.42%), C. albicans (74.90%) and P. aeruginosa (72.41%).
In general, the literature shows that Gram-positive bacteria are more susceptible to the action of PDT compared to Gramnegative bacteria. This is due to differences in the physiology of these microorganisms, since Gram-positive bacteria have a relatively porous outer membrane formed by a thicker layer of peptidoglycan and lipoteichoic acid14. This feature allowsa greater diffusion of the photosensitizer within the microbial cells,sincethey can be eliminated by various types of dye and lower doses of radiation, which explains the greater susceptibility of E. faecalis and S. aureusto PDT in this study.
On the other hand, the outer membrane of Gram-negative bacteria (Pseudomonas aeruginosa) is thinner and complex, being formed by a heterogeneous composition of proteins with porin function, lipopolysaccharides and lipoproteins that act as an effective barrier to limit the penetration of various substances14.
Regarding fungi, besides their nuclear membrane and increased cellular volume, they possess a cell wall composed of a thick layer of beta glucan and chitin, which promotes an intermediate permeability barrier between the Grampositive and Gram-negative bacteria32.
Variables such as exposure time and laser energy density, type and dye concentration influence the number of microorganisms affected by PDT12. In this study, a reduction in the number of CFU/mL C. albicans to 74.90% was achieved. On the other hand, de Souza et al.33 (2006) obtained a reduction of CFU/mL in a suspension of C. albicans to 88.6% when 0.1 mg/mL of methylene blue, 685 nm of laser light and an energy dose of 28 J/cm2 was used. The differences in results between these studies may be attributed to the dye concentration or to parameters used for laser irradiation.
In summary, despite the PDT not reducing the microorganisms completely, the results obtained lead to the conclusion that the treatment was able to promote the reduction of microbial cell viability using the selected parameters.
Acknowledgements
This study was supported by grants from Pernambuco State Foundation for Science and Technology- FACEPE (BIC- 0874-4.02/10) and CNPq - Brazil. The English version of this study has been revised by Sidney Pratt, Canadian, BA, MAT (The Johns Hopkins University), RSAdip (TEFL).
References
1. Siqueira JF Jr, Rôças IN, Favieri A, Lima KC. Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. J Endod. 2000; 26: 331-4. [ Links ]
2. Ricucci D, Siqueira JF Jr, Bate AL, Pitt Ford TR. Histologic investigation of root canal–treated teeth with apical periodontitis: a retrospective study from twenty-four patients. J Endod. 2009; 35: 493-502.
3. Siqueira JF Jr, Rôças IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod. 2008; 34: 1291-301.
4. Siqueira JF Jr. Endodontic infections: concepts, paradigms, and perspectives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 94: 281-93.
5. Câmara AC, Albuquerque MM, Aguiar CM, Correia ACRB. Antimicrobial activity of chlorhexidine in root canals instrumented with the ProTaper UniversalTM System. Braz J Oral Sci. 2010; 9: 402-9.
6. Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006; 32: 93-8.
7. Nóbrega LMM, Gadê-Neto CR, Dametto FR, Sarmento CFM, Carvalho RA. Ultrasonic irrigation in the removal of smear layer and Enterococcus faecalis from root canals. Braz J Oral Sci. 2011; 10: 221-5.
8. Case PD, Bird PS, Kahler WA, George R, Walsh LJ. Treatment of root canal biofilms of Enterococcus faecalis with ozone gas and passive ultrasound activation. J Endod. 2012; 38: 523-6.
9. Pinheiro SL, Araujo G, Bincelli I, Cunha R, Bueno C. Evaluation of cleaning capacity and instrumentation time of manual, hybrid and rotary instrumentation techniques in primary molars. Int Endod J. 2012; 45: 379-85.
10. Huth KC, Quirling M, Maier S, Kamereck K, Alkhayer M,Paschos E, et al. Effectiveness of ozone against endodontopathogenic microorganisms in a root canal biofilm model. Int Endod J. 2009; 42: 3-13.
11. Siqueira JF, Rôças IN. Optimising single-visit disinfection with supplementary approaches: a quest for predictability. Aust Endod J. 2011; 37: 92-8.
12. Gursoy H, Ozcakir-Tomruk C, Tanalp J, Yilmaz S. Photodynamic therapy in dentistry: a literature review. Clin Oral Investig. 2013; 17: 1113-25.
13. Kharkwal GB, Sharma SK, Huang YY, Dai T, Hamblin MR. Photodynamic therapy for infections: clinical applications. Lasers Surg Med. 2011; 43: 755-67.
14. Dai T, Huang Y, Hamblin MR. Photodynamic therapy for localized infections– state of the art. Photodiagnosis Photodyn Ther. 2009; 6: 170-88.
15. Valera MC, Maekawa LE, de Oliveira LD, Jorge AO, Shygei É, Carvalho CA. In vitro antimicrobial activity of auxiliary chemical substances and natural extracts on Candida albicans and Enterococcus faecalis in root canals. J Appl Oral Sci. 2013; 21: 118-23.
16. Garcez AS, Nuñez SC, Hamblim MR, Suzuki H, Ribeiro MS. Photodynamic therapy associated with conventional endodontic treatment in patients with antibiotic-resistant microflora: a preliminary report. J Endod. 2010; 36: 1463-6.
17. Bago I, Plecko V, Panduric DG, Schauperl Z, Baraba A, Anic I. Antimicrobial efficacy of a high-power diode laser, photo-activated disinfection, conventional and sonic activated irrigation during root canal treatment. Int Endod J. 2013; 46: 339-47.
18. Stojicic S, Amorim H, Shen Y, Haapasalo M. Ex vivo killing of Enterococcus faecalis and mixed plaque bacteria in planktonic and biofilm culture by modified photoactivated disinfection. Int Endod J. 2013; 46: 649-59.
19. Ok E, Ertas H, Saygili G, Gok T. Effect of photo-activated disinfection on bond strength of three different root canal sealers. Eur J Dent. 2014; 8: 85-9.
20. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965; 18: 340-8.
21. Sunde PT, Olsen I, Debelian GJ, Tronstad L. Microbiota of periapical lesions refractory to endodontic therapy. J Endod. 2002; 28: 304-10.
22. Ng R, Singh F, Papamanou DA, Song X,Patel C,Holewa C et al. Endodontic photodynamic therapy ex vivo. J Endod. 2011; 37: 217-22.
23. Garcez AS, Fregnani ER, Rodriguez HM, Nunez SC, Sabino CP, Suzuki H et al. The use of optical fiber in endodontic photodynamic therapy. Is it really relevant? Lasers Med Sci. 2013; 28: 79-85.
24. Komine C, Tsujimoto Y. A small amount of singlet oxygen generated via excited methylene blue by photodynamic therapy induces the sterilization of Enterococcus faecalis. J Endod. 2013; 39: 411-4.
25. Pupo YM, Gomes GM, Santos EB, Chaves L,Michel MD, Kozlowski VA Jr et al. Susceptibility of Candida albicans to photodynamic therapy using methylene blue and toluidine blue as photosensitizing dyes. Acta Odontol Latinoam. 2011; 24: 188-92.
26. Miyabe M, Junqueira JC, Costa AC, Jorge AO, Ribeiro MS, Feist IS. Effect of photodynamic therapy on clinical isolates of Staphylococcus spp. Braz Oral Res. 2011; 25: 230-4.
27. Foschi F, Fontana CR, Ruggiero K, Riahi R,Vera A,Doukas AG et al. Photodynamic inactivation of Enterococcus faecalis in dental root canals in vitro. Lasers Surg Med. 2007; 39: 782-7.
28. Queiroga AS, Trajano VN, Lima EO, Ferreira AF, Queiroga AS, Limeira FA Jr. In vitro photodynamic inactivation of Candida spp. by different doses of low power laser light. Photodiagnosis Photodyn Ther. 2011; 8: 332-6.
29. Pinheiro SL, Schenka AA, Neto AA, de Souza, CP, Rodriguez HM, Ribeiro MC. Photodynamic therapy in endodontic treatment of deciduous teeth. Lasers Med Sci. 2009; 24: 521-6.
30. Silva LA, Novaes AB, de Oliveira RR, Nelson-Filho P, Santamaria M, Silva RA. Antimicrobial photodynamic therapy for the treatment of teeth with apical periodontitis: a histopathological evaluation. J Endod. 2012; 38: 360-6.
31. Miranda RG, Santos EB, Souto RM, Gusman H, Colombo APV. Ex vivo antimicrobial efficacy of the EndoVac system plus photodynamic therapy associated with calcium hydroxide against intracanal Enterococcus faecalis. Int Endod J. 2013; 46: 499-505.
32. Pereira CA, Romeiro RL, Costa AC, Machado AK, Junqueira JC, Jorge AO. Susceptibility of Candida albicans, Staphylococcus aureus, and Streptococcus mutans biofilms to photodynamic inactivation: an in vitro study. Lasers Med Sci. 2011; 26: 341-8.
33. De Souza SC, Junqueira JC, Balducci I, Koga-Ito CY, Munin E, Jorge AO. Photosensitization of different Candida species by low power laser light. J Photochem Photobiol B. 2006; 83: 34-8.
 Correspondence:
Correspondence:
Bruna Paloma de Oliveira
Rua Mamanguape, 518, apto 2701
Boa Viagem - CEP: 51020250
Avenida Limeira 901, CEP: 13414-903
Recife, PE, Brasil
E-mail: bruna_paloma@msn.com
Received for publication: January 28, 2014
Accepted: March 20, 2014













