Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.13 no.1 Piracicaba Jan./Mar. 2014
ORIGINAL ARTICLE
Shear bond strength of a "solvent-free" adhesive versus contemporary adhesive systems
Eugenia Koliniotou-KoumpiaI; Pantelis KourosI; Effimia KoumpiaII; Maria Helvatzoglou-AntoniadesI
I Aristotle University of Thessaloniki, Dental School, Department of Operative Dentistry, Thessaloniki, Greece
II Aristotle University of Thessaloniki, Dental School, Department of Orthodontics, Thessaloniki, Greece
ABSTRACT
Aim: To compare the shear bond strength (SBS) of a solvent free self-etch adhesive with solvent containing adhesives. Methods: Forty-five human teeth were sectioned longitudinally to expose superficial dentin and substrates polished with 600-grit SiC paper. The adhesive area was isolated with a cylindrical Teflon mold 3x4 mm. Fifteen specimens were prepared for each material. Were evaluated a solvent free self-etch adhesive (Bond 1 SF), an ethanol self-etch adhesive (Futurabond M), and a water-acetone-ethanol self-etch adhesive (Optibond All-In-One). All specimens were subjected to an aging procedure by thermo-cycling (5000 cycles). Thirty-six specimens were stressed in shear at a rate of 0.5mm/min. Mean data values were analyzed statistically using the Welch robust analysis of variance and the Games-Howell statistic. Failure patterns were analyzed using stereomicroscope and scanning electron microscopy (SEM). Additional more dentin specimens were prepared for SEM. Results: The Bond 1 SF showed the statistically significant lowest SBS to dentin (Welch statistic p<0.001). Failures for Bond 1 SF were mainly adhesive failures with partial cohesive failures in the adhesive resin, while for Futurabond M and Optibond All-In-One were mainly mixed. SEM findings confirm the results. Conclusions: Eliminating solvents from self-etch adhesive systems may decrease the bonding strength to dentin.
Keywords: adhesives; bonding systems; shear bond strength; solvents.
Introduction
Clinically, the most attractive current adhesive systems are the one-step selfetch systems, since an additional rinse and drying step is no longer needed1.Selfetch adhesive systems are reported to exhibit low technique sensitivity regarding the conditions of the interface, which may be due to the fact that penetration of the acid components and the co-monomers occurs to the same depth2.
Self-etch dental adhesive systems are a complex mixture of components, including reactive monomers, an association of dissolved hydrophilic and hydrophobic monomers, cross linkers, initiators and solvents (such as water, acetone and ethanol)3. HEMA(2-hydroxyethyl methacrylate) is a widely used, water-soluble low-molecular-weight methacrylate monomer that enhances the penetration efficacy of an adhesive into demineralized dentin4,and has been reported to positively influence the bond strength to dentin5. HEMA also acts as a solvent and helps prevent hydrophilic and hydrophobic phase separations in one-step systems2,4,6-7.
Furthermore, adhesive systems also comprise organic molecules of lower polarity, which may form a homogeneous phase when proper co-solvents are used, such as acetone, ethanol or butanol8. Several researches have been carried out to determine the role of each ingredient found in adhesive systems2-4. Severalaspects have been studied, such as resin dentin bond strengths as a function of the nature, amount, and evaporation rate of incorporated solvents3,9. Authors have reported lower bonding effectiveness for simplified adhesive systems1,10. Recently, the presence and evaporation of solvents was determined to have an effect on monomer infiltration and polymerization9.Some authors have dealt with the effect of the solvent in the dentin primer of three-step adhesive systems on the bond strength and the marginal adaptation11.
However, there is no current study that compares the influence of a solvent free self-etch adhesive system versus an ethanol self-etch adhesive system and a water-acetoneethanol self-etch adhesive system. Therefore, the purpose of this laboratory study was to investigate the shear bond strength of a new solvent-free self-etch adhesive system versus two contemporary self-etch adhesive systems containing different solvents used on superficial dentin.
The null hypothesis tested was that self-etch adhesive systems containing different solvents or no solvent at all are equally effective in terms of shear bond strength when bonded to superficial dentin substrates.
Material and methods
Forty-five freshly extracted non-carious human third molars were stored in 0.5% chloramine solution at 4oC until use in the current study. All procedures detailed in the present investigation were performed in accordance with the protocol outlined by the Ethics Committee of the School of Dentistry, Aristotle University of Thessaloniki, Greece (Protocol 280/ 25.01.2012), regarding the recommended Standard Practices for Biological Investigations in extracted human teeth.
The roots of the teeth were removed 2 mm below the cemento-enamel junction using a water-cooled diamond saw. The crowns were sectioned perpendicularly to the tooth long axis, exposing the superficial dentin using low speed sectioning machine (Isomet 1000; Buehler Ltd, Lake Bluff, IL, USA). The exposed dentin substrates were abraded using 600-grit SiC paper, rinsed with water, and air dried for 3 s with dental air syringe at a distance of 15cm and air pressure of 3.8 kg/cm2. The specimens were randomly assigned to three groups in order to test shear bond strength of each material to dentin substrates. The dentin samples were embedded in a self-cure resin (Concise, 3M Dental Products, St Paul, MN, USA) in the middle of a metal ring mold. The surfaces to be tested were delimited using 3 mm diameter holes punched in teflon tape (PTFE teflon tape, CS Hyde Company, Inc. Illinois, USA) using a modified rubber-dam punch. This step was necessary to restrict the bonding of the adhesive system and the composite resin restorative material to the test area.
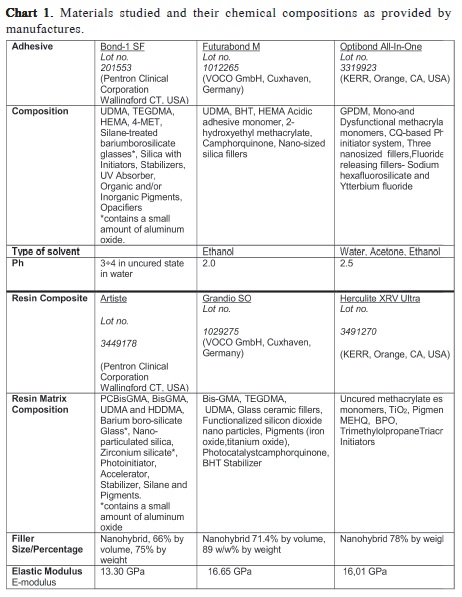
The adhesive systems tested were: A solvent free selfetch adhesive system - Bond 1 SF/Artiste (Pentron Clinical Corporation, Wallingford CT, USA), an ethanol self-etch adhesive system - Futurabond M /Grandio SO (Voco GmbH, Cuxhaven, Germany), and a water-acetone-ethanol self-etch adhesive system - Optibond All-In-One /Herculite Ultra XRV(KerrCorporation, Orange, CA, USA). The materials and their chemical compositions are listed in Chart 1. Twelve dentin samples were treated with each adhesive bonding system.
The adhesive systems were applied on the surfaces following the manufacturers' instructions. Bond-1 SF was applied in one coat, rubbing with the specific dip applicator for 20 s and then light cured for 10 sec. Futurabond M was applied with a brushing motion for 20 s in a single coat, thoroughly air-dried for at least 5 s to evaporate the solvent and light cured for 10 sec. Optibond All-In-One was applied in two coats by a brush with scrubbing motion for 20 s each, thoroughly air-dried for at least 5 s to evaporate the solvent and light cured for 10 s. After curing the adhesive systems, a polytetrafluoroethylene mold (3 mm in diameter and 4 mm in height) was placed over the specimen and filled with the respective manufacturer's composite resin in two increments. Each increment was light-cured for 40 s using a Bluephase (IvoclarVivadent AG, Schaan, Liechtenstein) device. This procedure resulted in cylindrical specimens of composite resin measuring 3 mm in diameter and 4 mm in height being bonded to the dentin. All specimens were subjected to an aging procedure by thermo-cycling (5000 cycles between 5 and 55oC, dwell time 30s). The shear bond strength (SBS) was determined after storage in water at room temperature (25oC) for 24 h.
Shear testing was conducted using a universal testing machine (Testometric AX, M 350-10KN, Rochdale, England) at a crosshead speed of 0.5 mm/min with the load of a metal chisel until fracture. The load at fracture, expressed in MPa, was calculated by dividing the peak load by the bonding area. The data were subjected to statistical analyses. The assumption of normality was tested with the Shapiro-Wilk test; which demonstrated normality with the data. Levene's test for equality of variances was used to test the homogeneity of variances, which was rejected. Accordingly, the hypothesis concerning the equality between mean data values was tested using the Welch robust analysis of variance. Pair wise comparisons between mean data values were conducted using the Games-Howell statistic. The analysis was performed with the SPSS 16.0 software and the statistical significance was set for p<0.05.
Failure patterns at the dentin/restorative system interface were analyzed under a stereomicroscope at 40 x magnification (Olympus Co, Tokyo, Japan) to determine the failure modes. Failure was considered to be: a) adhesive, if it occurred at the dentin/adhesive interface; b) cohesive, if it occurred in the material or in the substrate and c) mixed, when involving both the interface and the material. The bond failure patterns were not statistically analyzed. Three specimens of each fracture failure mode from the three groups tested for shear bond strength were randomly selected for evaluation using scanning electron microscopy (SEM). In order to investigate the effect of solvents on the adhesive interface, three more dentin specimens of each adhesive system were prepared for SEM examination. In these additional specimens, the processing followed the same procedures as described above, but the specimens were sectioned longitudinally to expose the dentin-adhesive junction. The SEM examinations were done on the longitudinally sectioned hybridized areas prepared in the proceeding procedures12.The sections were polished with No. 1200-grit paper discs and immersed in 6mol/L hydrochloric acid (HCl) for 30s. This was followed by immersion in a 1wt% sodium hypochlorite (NaOCl) for 10 min. Specimens were rinsed with distilled water and stored in desiccators overnight to dry. The specimens were then mounted on stubs, sputter-coated with carbon and examined by one evaluator under SEM (Jeol, J.S.M.-840 Tokyo, Japan) at 19 KV.
Results
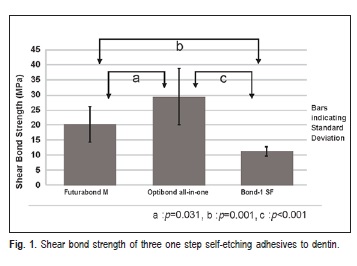
SBS test results are shown at Table 1. Statistically significant differences were found between the three materials (Welch statistic: F (2, 15.904)=30.863, p <0.001). The solvent free adhesive system (Bond 1 SF) exhibited the statistically significant lowest bond strength (11.3±1.54 MPa) when compared with the other two adhesive systems, Futurabond M (20.2±6.11 MPa) and Optibond All-In-One (29.2±9.35 MPa) (Games-Howell statistic, p=0.001 and p<0.001, respectively). Additionally, the bond strength of Futurabond M, was statistically significant lower than Optibond All-In-One (Games-Howell statistic, p=0.031). Optibond All-In-One showed the highest bond strength (Figure 1).
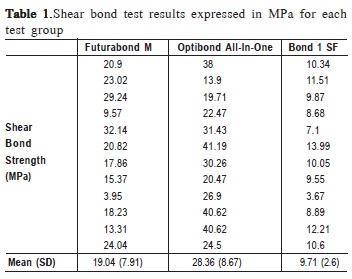
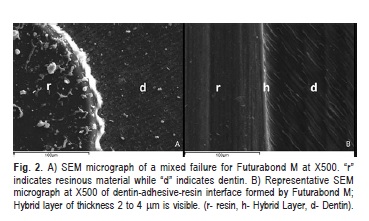
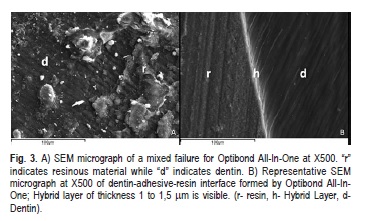
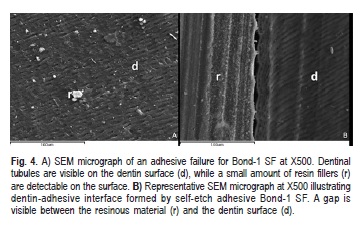
When analyzing failure patterns under a stereomicroscope and by SEM, it was possible to observe a variety of different zones of failure on the same surface. A mixed-failure (8/12) mode was predominantly observed in fractured specimens that were treated with Futurabond M (Figure 2A), the remaining fractures were adhesive (4/12). Failure modes in dentin/restorative material interface in fractured specimens that were treated with Optibond All- In-One were cohesive in dentin (4/12) or mixed (6/12) (Figure 3A), although two failures were adhesive (2/12). On the other hand, the groups treated with the solvent free adhesive (Bond-1 SF) exhibited a higher percentage of adhesive fractures (8/12) (Figure 4A) and some mixed failure modes (4/12).
SEM imaging of the dentin-adhesive interfaces formed by Futurabond M showed that the fiber network was entirely integrated into the adhesive layer (Figure 2B). The interface morphology can be described as similar to Optibond All- In-One, although it was not as uniform and the resin tags were more scattered and thinner. The hybrid layer thickness was approximately 1μm.
For the Optibond All-In-One adhesive system, the SEM images of the dentin–adhesive interfaces illustrated a uniform hybrid layer between 1-1.5μm thick. Optibond All-In-One delivers excellent and extensive penetration into dentinal tubules forming prominent tags. The junction between the adhesive and dentin appeared tight and continuous (Figure 3B).
Regarding the hybrid layer which forms Bond 1 SF, SEM analysis reveals an inconsistency. It appears that only parts of the material achieving contact with the dentin surface and there is a formation of arcs between the contact areas, which are probably due to the polymerization shrinkage of the material (Figure 4B).
Discussion
According to this study's results, the null hypotheses claiming that that self-etch adhesive systems containing different solvents or no solvent at all are equally effective in terms of shear bond strength when bonded to superficial dentin substrates has to be rejected.
Many factors can influence the bonding performance of adhesive systems to dentin, among these are: dentin substrate13, dentin treatment14 and chemical composition of the adhesive systems, including the solvents. Bond 1 SF is a light-cured, one-coat self-etch adhesive system in which the manufacturer has removed the solvent that is contained in virtually all other adhesive systems. All-in-one adhesive systems are currently composed of hydrophilic and hydrophobic monomers and solubilized in water and/or organic solvents, such as acetone or ethanol. Such monomers can penetrate the smear layer and perform a mild demineralization of the underlying dentin, forming covalent bonds with the dentin collagen and ionic bonds with hydroxyapatite15-16. A high solvent content, in particular water, is necessary for adequate ionization of the acidic monomer17.Increasing the water concentration reduces the monomer concentration and may reduce bond strengths, possibly by reducing the degree of monomer polymerization17.
While remembering that Bond 1 SF contains the 4-MET monomer, which was proven to form ionic bonds with hydroxyapatite15,it was reasonable to expect a certain degree of chemical interaction and to observe a shallow resin-dentin interface under SEM. SEM samples showed only partial formation of hybrid layer in this group. A gap was present between the material and the dentin substrate. The lack of a solvent in this adhesive system could be correlated with all facts described above and may explain the relatively lower bond strength of Bond 1 SF adhesive system. Additionally, the pH value of Bond 1 SF (pH=3-4) is comparatively higher than Optibond All-In-One (pH=2.5) and Futurabond M (pH- 1.4) and this may decrease the dentin demineralization depth and among other factors as the composition of the material and the infiltration capacity may lead to lack formation and lower bond strength. However, further studies are needed to support those hypotheses.
A previous study suggested that the presence of moisture on the dentin surface is essential when the Bond 1 Solvent Free adhesive system is applied18. Their results showed that the duration of air drying of the dentin surface affected microtensile bond strength and that prolonged air-drying of the dentin surface decreased the bond strengths. Although in this study the dentin surface was air dried prior adhesion for 3 sec, the solvent free adhesive system failed to achieve satisfactory bond strength values. Optibond All-In-One is a single component, light cure, one step self-etch adhesive system. The results of this current study showed that Optibond All-In-One revealed the highest shear bond strength values.
Optibond All-In-One values were statistically significantly higher than Futurabond M. The differences between these two one-step self-etch adhesive systems are attributed to the actual functional monomer and solvent system provided, consisting of a mixture of water, ethanol and acetone. The bonding performance obtained by self-etch adhesive systems varies considerably, depending on the composition and, more specifically, on the functional monomer included in the adhesive formulation19.The chemical composition of Optibond All-In-One adhesive system differs from the other adhesive systems examined in this present study due to its dimethacrylate monomers and GPDM (glycerol phosphate dimethacrylate). Glycerol phosphate dimethacrylate is a monomer that has a low viscosity and improved water solubility. The concentrations of individual monomers in the adhesive system and their interactions determine the extent of infiltration, ionization, and cross-linking obtained during polymerization and subsequent mechanical properties of the adhesive system16,19.It was determined that the ultra-structure of dentin-adhesive interfaces formed by self-etch adhesive systems depends on the interaction of functional monomers with dentin and on the acidity (pH=2.5) of the self-etch solution16,19. As mentioned in the results section, SEM images of the dentin–adhesive interfaces formed by Optibond All-In-One adhesive system illustrated a uniform hybrid layer and excellent penetration into dentinal tubules, forming prominent tags. The junction between the adhesive and dentin appeared tight and continuous.
In addition, several possible mechanisms may be responsible for the improved durability of resin-dentin bonds made with water-acetone-ethanol saturated dentin. It is possible that the ternary solvent system provides enhanced self-life stability and effective bond strength. Incomplete adhesive solvent removal following air-drying for all-in-one adhesive systems20-21, may impair polymerization22,and result in reduced bond strength23.The presence of acetone in Optibond All-In-One seems to help residual solvent and excess water removal following air-drying. Solvents and excess water need to be removed prior to or during monomer polymerization to obtain a pore free adhesive layer. Despite the presence of HEMA or hydrophilic groups in the final copolymer, reversible water uptake is inhibited by adequate crosslinking of the adhesive3. Solvents are used to dissolve polar and non-polar components3. Solvents containing the products examined in this current study showed large variations on shear bond strengths from one product to another. Evaporation of solvents may also contribute to the elimination of water from the hybrid layer precursor before polymerization3.
Furthermore, solvents modify pre-polymer viscosities and help adhesive spreading and substrate wetting3.Futurabond M is also an all in one self-etch adhesive system reinforced with nanoparticles. Manufacturers suggest that nano-scaled silicon dioxide particles with a diameter of ca. 20 nm (0.00002 mm) provided for cross-linking of the bond's resin components and improve its film building properties. The adhesive can thus optimally wet the released collagen fibers and micro-retentive etching pattern. As a result, the sensitive collagen fiber network cannot collapse and is entirely integrated into the adhesive layer. The far-reaching resin tags in the dentine tubules harden during polymerization and strengthen the retentive bond of the collagen fiber-bonding hybrid layer. The solvent system of this adhesive is ethanol, which is a polar solvent that forms hydrogen bonds. However, due to its much lower dielectric constant, ethanol is also a more appropriate solvent for less polar solutes7.
In Futurabond M, ethanol helps the adhesive to optimally wet the released collagen fibers in order to strongly bond to dentin24. This was confirmed by the results of this present study. The interface morphology can be described as similar to Optibond All-In-One, although it was not as uniform and resin tags were more scattered and thinner. This adhesive system also differs from Optibond All-In-One in that the hybrid layer was thinner. The long-term stability of adhesive restorations depends not only on the thickness of the hybrid layer, but also on the quality of the hybrid layer25.
Nonetheless, acetone is preferred as a solvent medium, due to its better hydrolytical stability of the functional monomers in acetone when compared to ethanol10.The reduced bonding effectiveness of Futurabond M can be assumed to be due to incorporating only ethanol, while Optibond All- In-One contains both ethanol and acetone. However the etching ability of the acidic monomers, which is included in each self-etch adhesive system and the contained polymerization initiators may be improving the bond strength to dentin16. The current results are in accordance with one in vitro study11 that examined the influence of acetone /water versus ethanol/water solvent mixture in a dentin primer of a three-step adhesive system. Those authors found that the marginal integrity of non-retentive composite fillings might be affected11. Those results also indicated that it is not only the water content of the primer that was a crucial factor, but also the type of organic solvent used (acetone or ethanol)11. Also the current results are in accordance with the results of a new study that evaluated the bonding effectiveness of this new solvent free adhesive system26.
Within the limitations of this study, it may be concluded that elimination of the solvent from a self-etch adhesive system may lead to decrease of infiltration of the adhesive components into the dental tissue's microstructures, debility of hybrid zone formation and eventually to a decrease of the bond strength to the dentin.
Acknowledgements
The kind help from the Physics Department, Scanning Electron Microscopy, Aristotle University of Thessaloniki, Greece and from Dr. Oikonomidis Stavros in performing SEM procedures for this present work is very much appreciated.
References
1. Sarr M, Kane AW, Vreven JJ, Mine A, Van Landuyt KL, Peumans M, et al.Microtensile bond strength and interfacial characterization of 11 contemporary adhesives bonded to bur-cut dentin. Oper Dent. 2010; 35: 94-104. [ Links ]
2. Moszner N, Salz U, Zimmermann J. Chemical aspects of self-etching enamel-dentin adhesives: a systematic review. Dent Mater. 2005; 21: 895-910.
3. Grégoire G, Dabsie F, Dieng-Sarr F, Akon B, Sharrock P. Solvent composition of one-step self-etch adhesives and dentine wettability. J Dent. 2011; 39: 30-9.
4. Nakabayashi N, Takarada K. Effect of HEMA on bonding to dentin. Dent Mater. 1992; 8: 125-30.
5. Nakaoki Y, Nikaido T, Pereira PN, Inokoshi S,Tagami J. Dimensional changes of demineralized dentin treated with HEMA primers. Dent Mater. 2000; 16: 441–6.
6. Van Landuyt KL, De Munck J, Snauwaert J, Coutinho E, Poitevin A, Yoshida Y, et al. Monomer-solvent phase separation in one-step self-etch adhesives. J Dent Res. 2005; 84: 183-8.
7. Van Landuyt KL, Snauwaert J, Peumans M, De Munck J, Lambrechts P, Van Meerbeek B. The role of HEMA in one-step self-etch adhesives. Dent Mater. 2008; 24: 1412-9.
8. Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007; 28: 3757-85.
9. Klein-Júnior CA, Zander-Grande C, Amaral R, Stanislawczuk R, Garcia EJ, Baumhardt-Neto R et al. Evaporating solvents with a warm airstream: effects on adhesive layer properties and resin-dentin bond strengths. J Dent. 2008; 36: 618-25.
10. Van Landuyt K, Peumans J, De Munck J, Lamprechts P, Van Meerbeek B. Extension of one-step self-etch adhesive into a multi-step adhesive. Dent Mater. 2006; 22: 533-44.
11. Balkenhol M., Huang J, Wöstmann B, Hannig M. Influence of solvent type in experimental dentin primer on the marginal adaptation of Class V restorations. J Dent. 2007; 35: 836-44.
12. Miyasaka K,& Nakabayashi N. Effect of Phenyl-P/HEMA acetone primer on wet bonding to EDTA-conditioned dentin. Dent Mater. 2001; 17: 499- 503.
13. Cavalcanti AN, De Souza ES, Lopes GDS, De Freitas AP, De Araújo RPC, Mathias P. Effect of a desensitizing dentifrice on the bond strength of different adhesive systems. Braz J Oral Sci. 2013; 12: 2.
14. Menezes FCH, Borges GA, Valentino TA, Oliveira MAHM, Turssi CP, Correr-Sobrinho L. Effect of surface treatment and storage on the bond strength of different ceramic systems. Braz J Oral Sci. 2009; 8: 119-23.
15. Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H et al. Comparative study on adhesive performance of functional monomers. J Dent Res. 2004; 83: 454-8.
16. Ikemura K, Kadoma Y, Endo T. A review of the developments of selfetching primers and adhesives. Effects of acidic adhesive monomers and polymerization initiators on bonding to ground smear layer-covered teeth. Dent Mater. J 2012; 30: 769-89.
17. Hiraishi N, Nishiyama N, Ikemura K, Yau JY, King NM, Tagami J et al. Water concentration in self-etching primers affects their aggressiveness and bonding efficacy to dentin. J Dent Res. 2005; 84: 653-8.
18.Takai T, Hosaka K, Kambara K, Thitthaweerat S, Matsui N, Takahashi M et al. Effect of air-drying dentin surfaces on dentin bond strength of a solvent-freeone-step adhesive. Dent Mater. J 2012; 31: 558-63
18. Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL. State of the art of self-etch adhesives. Dent Mater. 2011; 27: 17-28.
19. Sidhu SK, Omata Y, Tanaka T, Koshiro K, Spreafico D, Semeraro S, et al. Bonding characteristics of newly developed all-in-one adhesives. J Biomed Mater Res B. 2007; 80: 297-303.
20. Van Landuyt KL, Snauwaert J, De Munck J, Coutinho E, Poitevin A, Yoshida Y, et al. Origin of interfacial dropletswith one-step adhesives. J Dent Res. 2007;86: 739-44.
21. Nunes TG, Garcia FCP, Osorio R, Carvalho R, Toledano M. Polymerization efficacy of simplified adhesive systems studied by NMR and MRI techniques. Dent Mater. 2006; 22: 963-72.
22. Sadr A, Shimada Y, Tagami J. Effects of solvent drying time on micro shear bond strength and mechanical properties of two self-etching adhesive systems. Dent Mater. 2007; 23:1114-9.
23. Li F, Liu XY, Zhang L, Kang JJ, Chen JH. Ethanol-wet bonding technique may enhance the bonding performance of contemporary etch-and-rinse dental adhesives. J Adhes Dent. 2012; 14:113-20.
24. Eliguzeloglu E, Genc Ö, Özcopur B, Belli S, Eskitascioglu G, Özcan M. Influence of powdered dentin on the shear bond strength of dentin bondingsystems. Dent Mater J. 2012; 31: 758-64.
25. Shirban F, Khoroushi M, Shirban M. A new solvent-free one-step selfetch adhesive: bond strength to tooth structures. J Contemp Dent Pract. 2013; 14: 269-74.
 Correspondence:
Correspondence:
Eugenia Koliniotou-Koumpia
Department of Operative Dentistry
Dental School, Aristotle University of Thessaloniki
54124 Thessaloniki - GREECE
E-mail: jeny@dent.auth.gr
Received for publication: November 25, 2013
Accepted: March 10, 2014













