Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.13 no.1 Piracicaba Jan./Mar. 2014
ORIGINAL ARTICLE
In Vivo study of an intracanal dressing of calcium hydroxide/chlorhexidine in necrotic primary teeth
Juliana Oliveira GondimI; José Jeová Siebra Moreira NetoI; Débora Aline Silva GomesII; Elcilaine Rizzato AzevedoIII; Fabiano JeremiasIII; Elisa Maria Aparecida GiroIII
I Universidade Federal do Ceará - UFC, School of Pharmacy, Dentistry and Nursing, Department of Dental Clinics, Fortaleza, CE, Brasil
II Universidade Estadual Paulista - UNESP, Araraquara School of Dentistry, Department of Diagnosis and Oral Surgery, Araraquara, SP, Brasil
III Universidade Estadual Paulista - UNESP, Araraquara School of Dentistry, Department of Orthodontics and Pediatric Dentistry, Araraquara, SP, Brasil
ABSTRACT
Aim: To evaluate the clinical and radiographic success of endodontic treatment in human primary teeth with necrotic pulp with and without radiographically visible furcal/periapical lesion treated with a calcium hydroxide (CH) and chlorhexidine (CHX) intracanal dressing. The tested hypothesis was that there is no difference in the clinical and radiographic success in primary teeth medicated with CH pastes prepared with polyethylene glycol (PEG) or CHX. Methods: Thirty-two teeth with necrotic pulp were used in this randomized clinical study: 12 without and 20 with lesion. Canals were prepared and medicated with CH pastes with polyethylene glycol (CH/PEG) (n=16) or 2% CHX gel (CH/CHX) (n=16). Definitive filling was done after 30 days. The teeth were clinically and radiographically examined during 12 months to determine the success of the endodontic therapy. Data from clinical and radiographic examination of the initial condition and 12 months after treatment were compared using the Z test (α = 0.05). Results: There was no significant difference (p>0.05) in the success rate of teeth with and without lesion medicated with CH/PEG or CH/CHX. No significant difference (p>0.05) was found between the pastes regardless of the presence of lesion. Conclusions: Combination of CHX and CH was not more effective than the CH/PEG paste, as similar clinical and radiographic success rate was observed in teeth medicated with either type of intracanal dressing.
Keywords: chlorhexidine; primary teeth; calcium hydroxide.
Introduction
Infected root canals of teeth with chronic periapical lesions have a mixed endodontic microbiota with predominance of strict anaerobic microorganisms1-2, and the reduction or eradication of the bacterial infection in these teeth seems to be the most relevant factor for the success of endodontic therapy3. However, it has been demonstrated that complete elimination of microorganisms from the root canal system is not achieved with biomechanical preparation alone4-5. This fact is even more accentuated in primary teeth due to the limitations inherent to the instrumentation technique and the very peculiar internal anatomy of these teeth6-7. Therefore, intracanal medications are used to act against pathogens that resist after biomechanical preparation of the main canals and especially those lodged inside dentinal tubules, secondary canals and accessory canals2,4-5,8-9.
Calcium hydroxide (CH) is traditionally used in endodontics as the intracanal medication of choice for permanent teeth with pulp necrosis10, mainly because its highly alkaline pH (around 12.5) provides an excellent antibacterial activity4,11, and the capacity to inactivate bacterial endotoxin (LPS)12-13. However CH is not much effective against some bacterial species, especially Enterococcus faecalis11,14-15.
In order to increase the bactericidal efficacy of CH against resistant microorganisms, some authors have suggested its combination with chlorhexidine gluconate (CHX)2,16-18. This association has been suggested because CHX has a broad spectrum of activity against a wide array of oral microorganisms, including Gram-positive and Gram-negative bacteria15-17, high substantivity19 and capacity of inhibiting dentin matrix metalloproteinases20. However, the results of studies that evaluated the antibacterial efficacy of intracanal medications combining CH and CHX in permanent teeth are controversial4,16 and little research has been carried out in primary teeth2,14. Clinical trials are also important to evaluate the effect of this combination of medications in the endodontic treatment of primary teeth with pulp necrosis. The aim of this study was to evaluate the clinical and radiographic success of the endodontic treatment in human primary teeth with necrotic pulp with and without furcalD periapical lesion medicated with a CH/CHX paste as an intracanal dressing. The tested hypothesis was that there is no difference in the clinical and radiographic success in primary teeth medicated with CH pastes prepared with polyethylene glycol (PEG) or CHX.
Material and methods
This randomized blinded clinical study was approved by the Ethics Committee of the Araraquara School of Dentistry - UNESP (Protocol number 05/07). The sample consisted of 32 teeth from 28 patients of both genders aged 3 to 8 years who had been referred for dental treatment at the Pediatric Dentistry Clinic of the Araraquara Dental School, UNESP, Brazil.
Fulfillment of the following inclusion criteria was required for patient enrolment, based on clinical and radiographic examination: (a) healthy patients who had not used antibiotics or antimicrobials within the previous 3 months; (b) patients whose parents/caregivers agreed to sign an informed consent form authorizing their participation in the study; (c) anterior or posterior teeth with confirmed pulp necrosis due to caries (with or without furcal/periapical lesion), but with sufficient coronal structure to permit rubber dam isolation and further restoration and at least 2/3 of radicular remnant; (d) tooth mobility degree 0 or 1, and no periodontal pockets (periodontal probing depth < 3 mm); (e) no previous root canal intervention; (f) if present, the periapical lesion should not be invading the follicle of the germ of permanent successor. Children who presented any systemic alteration, were using antimicrobial mouthwashes, or had used antibiotics or antimicrobials within the previous 3 months, and those with behavioral problems were excluded.
The selected teeth were evaluated clinically and radiographically according to the following criteria: (a) painful symptomatology; (b) fistula; (c) gingival edema; (d) pathological tooth mobility; (e) radiolucent areas in the furcal/periapical region; (f) pathological root resorption. The radiographs were obtained using ultra-speed radiographic film in Han-Shin pediatric film holders (JON, São Paulo, SP, Brazil), according to the parallel technique with standardized exposure time and processing of exposed films.
After clinical and radiographic examination, the teeth were allocated to two groups, as follows: Group I: teeth with pulp necrosis without radiographically visible furcal/ periapical lesion (n=12); and Group II: teeth with pulp necrosis and radiographically visible radiolucent areas in the bone bifurcation/trifurcation region and/or periapical region suggestive of chronic periapical lesions (n=20).
The operative technique is described briefly as follows. The clinical procedures of endodontic treatment were performed by a single operator, who was an experienced pediatric dentist. First, antisepsis of the oral cavity was done with 0.12% CHX mouthwash (Farmácia Escola; UNESP, Araraquara, SP, Brazil). After infiltrative local anesthesia, placement of a rubber dam, cleaning of the tooth surface and operative field with 1% CHX (Farmácia Escola), the carious dentin tissue was removed with dentin excavators. New cleaning of the operative field with 1% CHX was done. Coronal access to root canals was prepared with air/water cooled high-speed spherical diamond burs (KG Sorensen Indústria and Comércio, São Paulo, SP, Brazil) followed by tapered safe-ended steel burs (Batt burs; Maillefer Instruments, Ballaigues, Switzerland).
Chemomechanical instrumentation of the canals was done according to the progressive neutralization technique using a sequence of three K-files (Maillefer Instruments) compatible with the canal diameter from the first instrument introduced in the canal combined with irrigation with 1.8 mL of 1% NaOCl at each change of file followed by suction of the solution. The working length was established 1.5-2 mm and 1 mm short of the radiographic apex in Groups I and II, respectively. The canals were dried with sterile absorbent paper points, filled with 16% ethylenediaminetetraacetic acid (EDTA pH 7.4; Biodinâmica Química and Farmacêutica Ltda., Ibiporã, PR, Brazil) for 3 minutes, followed by irrigation with 1% NaOCl, neutralized by 5% sodium thiosulfate, and a final flush with 2 mL of sterile saline, suction and drying with sterile absorbent paper points.
The root canals were filled with a CH-based intracanal medication chosen by lottery. In each group, half of the specimens (GI - n=6 and GII - n=10) received a paste prepared with CH p.a. (Merck, Darmstadt, Germany) and polyethylene glycol 400 (PEG 400; Labsynth Produtos para Laboratório LTDA, SP, Brazil), at 1:1 ratio (g/mL), and the other half received a paste prepared with CH p.a. and 2% CHX gel (Farmácia Escola) at 1:1 ratio (g/mL). The pastes were taken to the canals using lentulo spirals (Dentsply/ Maillefer). Complete filling of the canals was confirmed radiographically. The pulp chambers were cleaned and the access cavities were sealed with conventional glass ionomer cement (Ketac Fill; 3M ESPE, St. Paul, MN, USA).
After 30 days, under rubber dam isolation and aseptic conditions, the coronal seals were opened, and the intracanal medication was removed using the last instrument employed in the biomechanical preparation combined with sterile saline irrigation. The canals were dried and were obturated with a commercial CH-based paste [Calen®; S.S.White Artigos Dentários Ltda., Rio de Janeiro, RJ, Brazil; composition: 2.5 g CH, 0.5 g zinc oxide, 0.05 g colophony and 1.75 mL polyethylene glycol 400 (vehicle)] thickened with zinc oxide (S.S.White Artigos Dentários Ltda.). The root canal entrances were sealed with a layer of CH cement (Dycal; Dentsply Indústria and Comércio Ltda., RJ, Brazil). A glass ionomer cement base (Ketac Fill; 3M ESPE) was placed and the teeth were restored with either composite resin (Z250; 3M ESPE) or amalgam (Velvalloy; S.S.White Artigos Dentários Ltda.) for anterior and posterior teeth, respectively. A posttreatment periapical radiograph was taken.
The radiographic were obtained in a standardized manner (positioners Han-Shin), with focus distance / film of approximately 20 cm using dental X-ray apparatus (roping Dhabi Medical Industries Ltd. Dental – Ribeirão Preto, SP, Brazil), operating with 70KV and 10 mA, with an exposure time of 0.5 seconds. Film 0 or 2 Ultraspeed type (Eastman Kodak, Rochester, NY) were used. Disclosure of radiographs was performed using manual processing method time/ temperature in a developing solution.
Clinical and radiographic follow up was carried out 1, 3, 6 and 12 months after completion of the treatment. The clinical exams and the radiographic exposures were undertaken by the same operator who performed the endodontic treatment. The following clinical data were recorded: history of spontaneous pain, and presence of fistula or abscess, gingival edema and pathological mobility, suggestive of apical periodontitis. The radiographic aspects were presence of radiolucent areas in the furcal/periapical region and pathological root resorption, suggestive of apical periodontitis.
The clinical and radiographic data collected at the initial exam and at the 12-month postoperative follow up served as base to determine success or failure of the endodontic therapy. The radiographs were examined with the aid of a ×2 magnifying lens and a light box by two trained examiners blinded to the group to which the teeth belonged. In case of divergence, a consensus was reached between the examiners and a single opinion was considered.
The following criteria were reviewed to determine the success or failure of the endodontic therapy:
1. Complete repair (success)
• Clinically: absence of signs and symptoms suggestive of acute/chronic apical periodontitis, and
• Radiographically: absence of pathological root resorption; normal periodontal ligament space width; no development of furcal/periapical lesion, if no lesion was present at the beginning of the treatment; and total remission of lesion existing at the beginning of the treatment.
2. Incomplete repair (success)
• Clinically: absence of signs and symptoms suggestive of acute/chronic apical periodontitis, and
• Radiographically: absence of pathological root resorption and reduction of the furcal/periapical lesion size.
3. Absence of repair (failure)
• Clinically: signs and symptoms suggestive of acute/ chronic apical periodontitis and/or
• Radiographically: presence of pathological root resorption; no change in the size or increase of pre-existing furcal/periapical lesion during the follow-up period; or development of new furcal/periapical lesion.
Data from clinical and radiographic examination of the initial condition and 12 months after treatment were compared with respect to the presence/absence of radiographically visible furcal/periapical lesion and type of intracanal medication (CH/ PEG and CH/CHX), using the Z test for comparison of proportions with a significance level of 5%.
Results
From the 16 teeth that received CH/PEG as intracanal medication, 6 did not have and 10 had furcal and/or periapical lesion at the initial exam. Among the teeth without lesion, one was excluded from the sample due to trauma (avulsion) at 5 months of follow up and another presented apical resorption at 6 months of follow indicating failure of endodontic therapy. The other teeth of this group presented clinical and radiographic signs compatible with normality along the 12 months of evaluation. Among the teeth with lesion, 2 presented endodontic failure at 1 month posttreatment, as one of them presented a fistula and the other presented swelling, pain and redness together with abundant purulent secretion through the radicular canal. The other teeth with lesion presented absence of signs and symptoms suggestive of acute apical periodontitis and the radiographic findings were partial/complete remission of furcal/periapical lesion size and absence of pathological root resorption along the 12 months of evaluation.
The other half of the sample (16 teeth - 6 without and 10 with furcal/periapical lesion) received CH/CHX as intracanal medication. From the teeth without lesion at the initial exam, 1 presented radiographic failure at 3 months, with widening of the periodontal ligament space and pathological root resorption. The other teeth without lesion presented clinical and radiographic signs compatible with normality along the 12 months of evaluation. From the teeth with lesion at the initial exam, 2 presented endodontic failure. One tooth was clinically normal at 3 months posttreatment, but the radiographic examination revealed widened periodontal ligament space and development of a periapical lesion associated with the mesial root. The other tooth presented pathological mobility, root resorption and increase of lesion size at 6 months posttreatment. The other 8 teeth with lesion presented absence of clinical signs and symptoms, partial/complete remission of the radiographically visible furcal/periapical lesion and absence of pathological root resorption along the 12 months of evaluation.
Table 1 presents the clinical and radiographic success rate for each group after a follow-up period of 12 months. There was no statistically significant difference (p>0.05) in the clinical and radiographic success rate of teeth without furcal/periapical lesion medicated with CH/PEG or CH/CHX paste. No statistically significant difference (p>0.05) was observed in the clinical and radiographic success rate of teeth with lesion medicated with CH/PEG or CH/CHX pate. Comparing the intracanal medications (CH/PEG and CH/ CHX) no statistically significant difference (p>0.05) was found regardless of the presence or absence of lesion.
Table 2 presents the number of primary teeth with necrotic pulp without radiographically visible furcal/periapical lesion at the initial exam, which resulted in endodontic success or failure at 1-, 3-, 6- and 12month posttreatment periods according to the type of intracanal medication.

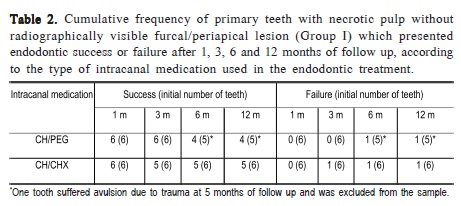

Table 3 presents the number of primary teeth with necrotic pulp and radiographically visible furcal/periapical lesion at the initial exam, which exhibited success (partial/total lesion repair) or failure of the endodontic therapy at 1-, 3-, 6- and 12-month posttreatment periods according to the type of intracanal medication.
Discussion
The endodontic treatment of teeth with infected root canals and necrotic pulp aims at eradicating the microorganisms from the root canal system by combining chemomechanical preparation and use of an intracanal medication5,8-9,21. Considering the nature of the endodontic infection and the peculiarities of primary teeth (complex root canal system, physiological root resorption, and risk of damage to the germ of permanent successor), in the same way as observed in permanent teeth, biomechanical preparation alone cannot eliminate the microorganisms from the main root canals and canals ramifications of these teeth3,5 and the use of an intracanal dressing is necessary for an auxiliary action. Over the last years, there has been a shift in the paradigms of endodontic treatment in primary teeth, and biomechanical preparation of these teeth followed by use of an intracanal medication has become ever more frequent9,14,21-22.
CH is one of the materials of choice for medication of infected root canals of permanent and primary teeth3,9,21-22. Because of its high alkalinity (pH above 12), several biological properties are attributed to CH, including bactericidal action, compatibility with biological tissues, inactivation of bacterial endotoxin, inhibition of pathological root resorption, and stimulation of mineralized tissue deposition with consequent repair of the area5,11,13,23.
The majority of microorganisms in the root canals of teeth with necrotic pulp cannot survive in the highly alkaline environment produced by CH11,150. However, its low bactericidal action against E. faecalis11,15 led some authors to propose the combination of CHX with CH in order to produce an intracanal medication with higher antibacterial efficacy and thus increase the chances of endodontic success 11,24-25. There is no consensus in the literature regarding the ideal concentration of CHX to be added to CH-based intracanal medications2,14,17,25 and very few studies on endodontic treatment in primary teeth are available2,14.
While some studies14,23-24 have demonstrated that the antibacterial effect of CH is significantly increased with the addition of CHX, others4,16,25 have reported that the antimicrobial efficacy seems to be reduced when this substance is combined with CH. The lack of an additional antibacterial action observed in this study when CHX was incorporated to CH may be attributed to different reasons. CH and a CHX act at different pH levels; CHX has a pH-dependent antibacterial activity, with optimal pH ranging from 5.5 and 7.0, and tends to be instable and precipitate in highly alkaline environments11,17. Therefore, it is suggested that the difference of pH of action may interfere in the intrinsic response of each substance. In addition, despite the high substantivity of CHX17,19 and although residues of CH pastes, regardless of the vehicle, remain inside the dentinal tubules even after removal of the intracanal medication18, CHX is capable of interacting with dentin components, which also limits its action26.
In the present study, the combination of these substances was not more effective than the CH/PEG dressing, regardless of the presence or absence of furcal/periapical lesion. Similar results have been reported in permanent teeth4. Conversely, Onçag, Gogulu and Uzel14 (2006) had better results using CHX alone or in combination with CH in a clinical trial with primary teeth with necrotic pulp.
The clinical and radiographic evaluation of primary teeth with pulp necrosis along a 12-month period revealed low endodontic failure rate without significant difference between teeth with and without radiographically visible furcal/ periapical lesion medicated with CH/PEG or CH/CHX and obturated with CH/PEG paste. In an overview of the results, 4 of 20 teeth with lesion (20%) presented failure of the endodontic therapy, with complete and partial repair of the lesion area in 55% and 25% of the teeth, respectively. In a clinical and radiographic study over a 12-month period, Ercan, et al.16 (2007) evaluated the effect of intracanal medication with CH and CHX in permanent teeth with periapical lesions needing endodontic retreatment and found 64% of complete repair, 14% of partial repair and 22% of failure. Coser , Gondim and Giro21 (2008) evaluated the effect of CH in the treatment of primary teeth with necrotic pulp and bone loss in the furcal region over 4 years and found a significant reduction or complete remission of the lesion within the first 12 months of follow up.
Partial reduction of lesion size or persistence of clinical signs and symptoms even after sufficient posttreatment time for the occurrence of complete lesion repair may be due to the resistance of residual microorganisms, but little research has been done on this subject in primary teeth3,27-28. Because of the current lack of consensus about the best intracanal medication for primary teeth with necrotic pulp, different substances have been used, causing confusion especially among less-experienced professionals. Consequently, laboratory and clinical studies should be encouraged in the continuous search for the ideal intracanal medication for primary teeth, considering their peculiarities and the behavioral conditions of the pediatric patient. This would represent an important step forward to establish a standardized protocol for the endodontic treatment of primary teeth with necrotic pulp to be adopted and disseminated by dental schools
Therefore, combination of CHX and CH was not more effective than the conventional CH paste with PEG for use as intracanal dressing in primary teeth with necrotic pulp with and without furcal/periapical lesion, as both medications yielded similar clinical and radiographic success of the endodontic therapy after 12 months of follow up.
Acknowledgements
This study was supported by grants from The São Paulo State Research Foundation (Grant # 07/54433-4 – financial support; Grant #2007/01183-0 – Doctorate scholarship).
References
1. Cogulu D, Uzel A, Oncag O, Eronat C. PCR-based identification of selected pathogens associated with endodontic infections in deciduous and permanent teeth.Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106: 443-9. [ Links ]
2. Ito IY, Junior FM, Paula-Silva FW, Da Silva LA, Leonardo MR, Nelson- Filho P. Microbial culture and checkerboard DNA-DNA hybridization assessment of bacteria in root canals of primary teeth pre- and post-endodontic therapy with a calcium hydroxide/chlorhexidine paste. Int J Paediatr Dent. 2011; 21: 353-60.
3. Sakamoto M, Siqueira Jr JF, Rôças IN, Benno Y. Bacterial reduction and persistence after endodontic treatment procedures. Oral Microbiol Immunol. 2007; 22: 19-23.
4. Manzur A, Gonzalez AM, Pozos A, Silva-Herzog D, Friedman S. Bacterial quantification in teeth with apical periodontitis related to instrumentation and different intracanal medications: a randomized clinical trial. J Endod. 2007; 33: 114-8.
5. Vera J, Siqueira JF Jr, Ricucci D, Loghin S, Fernández N, Flores B, et al. One- versus two-visit endodontic treatment of teeth with apical periodontitis: a histobacteriologic study. J Endod. 2012; 38: 1040-52.
6. Simpson WJ. An examination of root canal anatomy of primary teeth. J Can Dent Assoc .1973; 39: 637-40.
7. Aminabadi NA, Farahani RM, Gajan EB. Study of root canal accessibility in human primary molars. J Oral Sci. 2008; 50: 69-74.
8. Paredes-Vieyra J, Enriquez FJ. Success rate of single- versus two-visit root canal treatment of teeth with apical periodontitis: a randomized controlled trial. J Endod. 2012; 38: 1164-9.
9. Lima RA, Carvalho CB, Ribeiro TR, Fonteles CS. Antimicrobial efficacy of chlorhexidine and calcium hydroxide/camphorated paramonochlorophenol on infected primary molars: a split-mouth randomized clinical trial. Quintessence Int. 2013; 44: 113-22.
10. Lee LW, Hsiao SH, Chang CC, Chen LK. Duration for apical barrier formation in necrotic immature permanent incisors treated with calcium hydroxide apexification using ultrasonic or hand filing. J Formos Med Assoc. 2010; 109: 596-602.
11. Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011; 44: 697-730.
12. Oliveira LD, Carvalho CA, Carvalho AS, Alves Jde S, Valera MC, Jorge AO. Efficacy of endodontic treatment for endotoxin reduction in primarily infected root canals and evaluation of cytotoxic effects. J Endod. 2012; 38: 1053-7.
13. Adl A, Motamedifar M, Shams MS, Mirzaie A. Clinical investigation of the effect of calcium hydroxide intracanal dressing on bacterial lipopolysaccharide reduction from infected root canals. Aust Endod J. 2013 Dec 13. doi: 10.1111/ aej.12054. [Epub ahead of print]
14. Onçag O, Gogulu D, Uzel A. Efficacy of various intracanal medicaments against Enterococcus faecalis in primary teeth: an in vivo study. J Clin Pediatr Dent. 2006; 30: 233-7.
15. de Lucena JM, Decker EM, Walter C, Boeira LS, Löst C, Weiger R. Antimicrobial effectiveness of intracanal medicaments on Enterococcus faecalis: chlorhexidine versus octenidine. Int Endod J. 2013; 46: 53-61.
16. Ercan E, Dalli M, Dulgergil CT, Yaman F. Effect of intracanal medication with calcium hydroxide and 1% chlorhexidine in endodontic retreatment cases with periapical lesions: an in vivo study. J Formos Med Assoc. 2007; 106: 217-24.
17. Mohammadi Z, Abbott PV. The properties and applications of chlorhexidine in endodontics. Int Endod J. 2009; 42: 288-302.
18. da Silva JM, Andrade Junior CV, Zaia AA, Pessoa OF. Microscopic cleanliness evaluation of the apical root canal after using calcium hydroxide mixed with chlorhexidine, propylene glycol, or antibiotic paste. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 111: 260-4.
19. Khademi AA, Mohammadi Z, Havaee A. Evaluation of the antibacterial substantivity of several intra-canal agents. Aust Endod J. 2006; 32: 112-5.
20. Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, et al. Strategies toprevent hydrolytic degradation of the hybrid layer-A review. Dent Mater. 2013; 29: 999-1011.
21. Coser RM, Gondim JO, Giro EMA. Evaluation of 2 endodontic techniques used to treat human primary molars with furcation radiolucency area: A 48- month radiographic study. Quintessence Int. 2008; 39: 549-57.
22. de Sousa DL, de Sousa RB, Pinto DN, Neto JJ, de Carvalho CB, de Almeida PC. Antibacterial effects of chemomechanical instrumentation and calcium hydroxide in primary teeth with pulp necrosis. Pediatr Dent. 2011; 33: 307-11.
23. Gomes BP, Montagner F, Berber VB, Zaia AA, Ferraz CC, de Almeida JF et al. Antimicrobial action of intracanal medicaments on the external root surface. J Dent. 2009; 37: 76-81.
24. Gomes BP, Vianna ME, Sena NT, Zaia AA, Ferraz CC, de Souza Filho FJ. In vitro evaluation of the antimicrobial activity of calcium hydroxide combined with chlorhexidine gel used as intracanal medicament. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 102: 544-50.
25. Oliveira JC, Alves FR, Uzeda Md, Rôças IN, Siqueira JF Jr. Influence of serum and necrotic soft tissue on the antimicrobial effects of intracanal medicaments. Braz Dent J. 2010; 21: 295-300.
26. Portenier I, Haapasalo H, Orstavik D, Yamauchi M, Haapasalo M. Inactivation of the antibacterial activity of iodine potassium iodide and chlorhexidine digluconate against Enterococcus faecalis by dentin, dentin matrix, type-I collagen, and heat-killed microbial whole cells. J Endod. 2002; 28: 634-7.
27. Siqueira Jr JF. Microbial causes of endodontic flare-ups. Int Endod J. 2003; 36: 453-63.
28. Soares JA, Leonardo MR, Silva LAB, Tanomaru Filho M, Ito IY. Effect of biomechanical preparation and calcium hydroxide pastes on the antisepsis of root canal systems in dogs. J Appl Oral Sci. 2005; 13: 93-100.
 Correspondence:
Correspondence:
Juliana Oliveira Gondim
Av. Cel Miguel Dias, 372 - Edson Queiroz
CEP: 60810-160 - Fortaleza, CE, Brazil
E-mail: jujugondim@yahoo.com.br
Received for publication: February 07, 2014
Accepted: March 20, 2014













