Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.13 no.3 Piracicaba Jul./Set. 2014
ORIGINAL ARTICLE
Quality of life in temporomandibular disorder patients with localized and widespread pain
Maísa Soares GuiI; Marcele Jardim PimentelII; Marta Cristina da Silva GamaI; Glaucia Maria Bovi AmbrosanoIII; Célia Marisa Rizzatti BarbosaII
I Universidade Estadual de Campinas - UNICAMP, Piracicaba Dental School, Department of Anatomy, Piracicaba, SP, Brazil
II Universidade Estadual de Campinas - UNICAMP, Piracicaba Dental School, Department of Prosthesis and Periodontology, Piracicaba, SP, Brazil
III Universidade Estadual de Campinas - UNICAMP, Piracicaba Dental School, Department of Community Dentistry, Area of Statistics, Piracicaba, SP, Brazil
ABSTRACT
Aim: To compare temporomandibular (TMD) subgroups classified according to the presence of localized pain (LP) or widespread pain (WP) in order to assess the quality of life domains and verify which components affect most the functional capacity of facial pain patients. Methods: A cross-sectional study was conducted and the Short Form-36 Health Survey was applied in order to assess quality of life. Thirty-nine TMD/WP patients, 37 TMD/LP patients and 40 subjects free of TMD complaints were evaluated. Results: TMD/WP patients differed significantly from healthy controls in all SF-36 components and TMD/LP patients ranked between them. It was also observed that patients with bodily pain and TMD with WP are respectively, 4.16 and 49.42 times more likely to have low functional capacity. Conclusions: Functional capacity in TMD subgroups was only affected by the presence of bodily pain and WP. These patients feature high chance of low functional capacity. Furthermore, TMD patients with localized and widespread pain share roleemotional impairments.
Keywords: facial pain; temporomandibular joint dysfunction syndrome; quality of life.
Introduction
Temporomandibular disorders (TMD) are defined as a set of conditions affecting the masticatory muscles or joints and exhibiting pain as their primary characteristic1-2. It has been described that individuals with TMD could display diffuse hyperalgesia and allodynia3-4 and it was suggested that they have a fundamental problem with pain or sensory processing rather than an abnormality confined to a specific region of the body where pain is perceived to originate5-6.
Recently, two TMD clinical subgroups were proposed based on findings showing a group of TMD patients that was split with respect to patients' tender point score (one of the diagnosis criteria for fibromyalgia) into an insensitive subgroup resembling healthy control subjects and into a sensitive subgroup resembling patients with fibromialgia1. The distinction between localized and generalized pain in TMD patients was recognized as important for both, patient diagnosis and for proper understanding of the etiology and pathophysiology of chronic pain4,7.
Numerous psychological and behavioral factors are well-established influences on a wide range of pain conditions including TMD pain2. An orofacial pain prospective study identified that psychological and behavioral factors have become significant influences on TMD pain8. Another study also supported the interpretation that psychosocial parameters may be independent predictors for the development of chronic pain conditions and their generalization1.
The Short-form-36 is a global health-related quality of life measurement9 that could help identify the similarities and differences in those TMD patients. Indeed, the knowledge of how physical and mental components influence the quality of life and how people realize these events in their life has increased regarding the etiology of chronic pain. Previous studies clearly demonstrated the psychological process, i.e., emotion could modulate pain and vice-versa10-11.
The aim of this study was to compare TMD subgroups that were classified according to the presence of localized or widespread pain in order to assess the quality of life domains and to verify which components most affect the functional capacity of facial pain patients.
Material and methods
Study design
A cross-sectional study was conducted in pain-free healthy subjects and two subgroups of TMD patients recruited from the clinic of the Piracicaba Dental School/UNICAMP and the communities surrounding the school between January 2010 and November 2012.
Ethical Procedures
This study was approved by the Ethics Committee on Research Involving Human Subjects under protocol number 103/ 2009. After a verbal presentation of the project, the volunteers signed an informed consent form to participate in the study.
Participants
For TMD case, patients with myogenic facial pain diagnosed using the Research Diagnostic Criteria for TMD (RDC/TMD)12 were invited to participate. Calibrated examiners performed the RDC/TMD clinical examination on all subjects. The inclusion criteria were gender (female), due to the higher prevalence of TMD and longer duration of the condition in women13-14 and presence of symptomatic TMD. Exclusion criteria were the presence of systemic diseases, polyarthritis, exposure to macro facial trauma, dislocated joints, use of orthodontic braces, dental pain, and the presence of sinusitis, ear infections, cancer and hormonal disorders.
After that, subgroups of TMD patients were defined according to the presence or absence of widespread pain, palpation tenderness. The subgroup with widespread pain (TMD/WP) was identified on the basis of their tender point count, which is an easy practicable screening tool for those patients1. Briefly, widespread pain was present when the palpation of 18 body sites elicited pain at diagonally opposite quadrants of the body (i.e., above and below the waist, on both the left and right sides)1,7. Three pounds of digital palpation pressure were applied bilaterally for 2 s to each site by calibrated examiners. At each location, a response of pain to palpation was recorded as tenderness. The TMD patients without widespread pain were classified as having "localized pain" (TMD/LP subgroup).
Control subjects had neither TMD nor widespread pain classification. Then, a control group of female individuals without complaint, which were free of any bodily or facial pain condition, was also recruited and invited to participate.
Variables and Data Sources
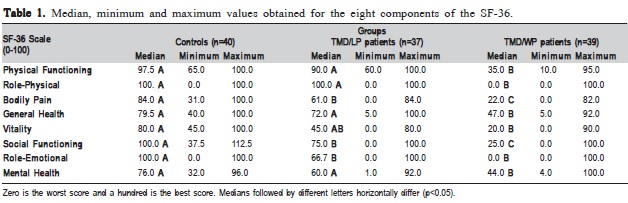
Quality of life was assessed by a generic multidimensional instrument: The Short Form-36v2® Health Surveys (SF-36)9. Briefly, this questionnaire measures eight health domains: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional and mental health9. The score for each scale varies from 0 to 100, and the higher score corresponds to better life and provides psychometricallybased physical component summary (PCS) and mental component summary (MCS) scores.
The mental health measure has been shown to be useful in screening for psychiatric disorders, as has the MCS measures15. The MCS had a sensitivity of 74% and a specificity of 81% in detecting patients diagnosed with depressive disorder15. The SF-36 has been widely used in research with excellent metric properties (sensitivity, validity and reliability)16 and it was translated and validated for the Portuguese language17.
Statistical Methods
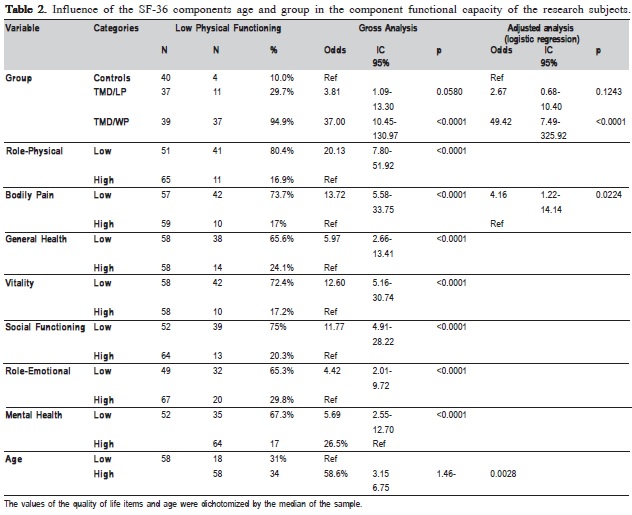
Data were analyzed by Kruskal Wallis and Dunn considering a significance level of 5%. The values of life quality items and age were dichotomized by the median of the sample. Bivariate analysis was performed by associating each variable with functional capacity. Variables with p<0.20 in bivariate analysis were tested in a multiple logistic regression model, remaining in the model those with p<0.05.
Results
From the initial sample size of 120 subjects (40 per group), one TMD/WP patient was lost to follow up and three TMD/LP patients withdrew from the study. Therefore, 40 TMD-free healthy controls (mean age 50.93±12.34), 37 TMD/LP patients (mean age 24.92±5.0) and 39 TMD/WP patients (mean age 53.21±9.34) were evaluated. There was statistically significant difference (p<0.001) in the mean age of TMD/LP patients when compared with TMD/WP patients and controls, possibly because localized facial pain appears earlier than widespread facial pain.
The main result of this study is that TMD/WP patients significantly differ from healthy controls in all components, while TMD/LP patients ranked in between (Table 1). However, the emotional factors did not differ between TMD subgroups and general health, mental health, physical function and role-physical domains were not different between TMD/LP patients and controls.
Regardless of the other variables it could also be observed that patients with more bodily pain and widespread pain are, respectively, 4.16 and 49.42 times more likely to have lower functional capacity than healthy controls (Table 2).
Discussion
The quality of life components of TMD/LP patients ranked in between TMD/WP patients and healthy controls. Furthermore, only the presence of widespread pain and bodily pain affected the functional capacity of the individual.
The present results also show that role-emotional (problems with work or other daily activities as a result of emotional problems) is not significantly different between TMD subgroups and could represent a common point that differentiates them from the control group. However, there was a great difference between patients with localized and widespread pain (respectively, 66.7 and 0).
One of the limitations of the study, is the fact that this was cross-sectional research and temporal conclusions cannot be drawn (e.g., it is unknown if the emotional problems occurred before or after pain). In addition, there is a lack of control group matching with the TMD/LP subgroup with respect to age.
Potential psychosocial risk factors for chronic TMD were identified, revealing components constructs as stress and negative affectivity, global psychosocial symptoms, passive pain coping, and active pain coping8 that provide evidences of associations between psychosocial factors and TMD.
Furthermore, strong support was provided for the hypothesis that chronic widespread pain is one manifestation of the somatization process, which was described as the expression of personal and social distress through physical symptoms18, and high nocturnal masseter muscle activity was related to higher intensity of headache and higher somatization in TMD patients19. However, it was previously described that TMD subgroups ("sensitive" with generalized increase of evoked pain, and "insensitive" with localized pain complaint) did not differ with respect to psychological parameters and sensitive TMD had shorter pain duration than fibromyalgia patients1.
In general, painful stimuli elicit considerable cognitive and emotional activity in the brain20. The notion that widespread pain syndromes, as fibromyalgia, might represent generalized neurobiological amplification of sensory stimuli has some support from functional imaging studies suggesting that the insula is the most consistently hyperactive neurocortical region of the pain matrix. This region has been noted to play a critical role in sensory integration, with the posterior insula having a more exclusive sensory role, and the anterior insula being associated with the emotional processing of sensations 5.
Another recent study demonstrated that rejection and physical pain are similar not only in that they are both distressing, but also they share the same common somatosensory representation 21.
Emotional modulation of muscle pain was associated with polymorphisms in the serotonin-transporting gene and indicated that polymorphisms that lead to a high expression of the serotonin transporter gene are highly associated with the ability to modulate deep types of pain in relation to the emotional state. Further, only the studied participants with a high expression of serotonin transporter experienced a signiûcantly changed perception of jaw muscle pain depending on their emotional state22. Taken together, all these factors seem to indicate that emotional characteristics could be predisposing factors of these chronic facial pain conditions.
Despite the age difference, Physical Function and Role- Physical domains did not differ between TMD/LP patients and healthy controls. Low physical functioning was considerably more related to TMD with widespread pain, which means very limited in performing all physical activities, including bathing or dressing 15.
It could be related to pain but, particularly, with helplessness and small practice of pain coping. This refers to a belief that nothing can be done to solve a problem, characterized by emotional, motivational, and cognitive deficits23. While positive emotions lead to pain reduction10, pain catastrophizing may lead to hyperalgesia via independent processes of spinal nociception, perhaps related to the subjective evaluation of pain (e.g., memory, attention) 24.
The present findings also show that the distinction between localized and generalized pain in TMD is important both for patient diagnosis and for treatment target. Since psychosocial factors play a role in the pathogenesis of musculoskeletal pain25, the knowledge of TMD subgroups characteristics and their functional impairments could help to target treatment approach.
It may be concluded that functional capacity in the TMD subgroups was only affected by the presence of bodily pain and widespread pain. These patients feature high chance of having low functional capacity. Furthermore, TMD/LP and TMD/WP patients shared role-emotional impairments.
Acknowledgements
Coordination of Improvement of Higher Education Personnel – CAPES, for a two-years post-graduation sponsorship (#2009-2010).
References
1. Pfau DB, Rolke R, Nickel R, Treede RD, Daublaender M. Somatosensory profiles in subgroups of patients with myogenic temporomandibular disorders and fibromyalgia syndrome. Pain. 2009; 147: 72-83. [ Links ]
2. Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, et al. Clinical findings and pain symptoms as potential risk factors for chronic TMD: Descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011; 12: T27-45.
3. Smith MT, Wickwire EM, Grace EG, Edwards RR, Buenaver LF, Peterson S, et al. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009; 32: 779-90.
4. Slade GD, Smith SB, Zaykin DV, Tchivileva IE, Gibson DG, Yuryev A, et al. Facial pain with localized and widespread manifestations: Separate pathways of vulnerability. Pain. 2013; 154: 2335-43.
5. Williams DA, Clauw DJ. Understanding fibromyalgia: Lessons from the broader pain research community. J Pain. 2009; 10: 777-91.
6. Weissman-Fogel I, Moayedi M, Tenenbaum H, Goldberg M, Freeman B, Davis K. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain. 2011; 152: 384-96.
7. Chen H, Slade G, Lim PF, Miller V, Maixner W, Diatchenko L. Relationship between temporomandibular disorders, widespread palpation tenderness, and multiple pain conditions: A case-control study. J Pain. 2012; 13: 1016-27.
8. Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, Bair E, et al. Potential psychosocial risk factors for chronic TMD: Descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011; 12: T46-60.
9. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992: 473-83.
10. Rhudy JL, Meagher MW. The role of emotion in pain modulation. Curr Opin Psychiatr. 2001; 14: 241-5.
11. Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000; 288: 1769-72.
12. Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: Review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992; 6: 301-55.
13. Gonçalves DA, Dal Fabbro AL, Campos JA, Bigal ME, Speciali JG. Symptoms of temporomandibular disorders in the population: an epidemiological study. J Orofac Pain. 2010; 24: 270-8.
14. LeResche L, Drangsholt M. Temporomandibular disorders. In: Goldman MB, Troisi R, Rexrode KM, editors. Women and health. 2.ed. London: Academic Press; 2013. p.1367-78.
15. Ware JE, Kosinski M, Keller S. SF-36 physical and mental health summary scales: A user's manual. Health Assessment Lab; 1994.
16. Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T, Mishima M. A comparison of the responsiveness of different generic health status measures in patients with asthma. Qual Life Res. 2003; 12: 555-63.
17. Ciconelli RM, Ferraz MB, Santos W, Meinão I, Quaresma MR. Brazilian- Portuguese version of the SF-36. A reliable and valid quality of life outcome measure. Rev Bras Reumatol. 1999; 39: 143-50.
18. McBeth J, Macfarlane GJ, Benjamin S, Silman AJ. Features of somatization predict the onset of chronic widespread pain: Results of a large population based study. Arthritis Rheum. 2001; 44: 940-6.
19. Shedden Mora M, Weber D, Borkowski S, Rief W. Nocturnal masseter muscle activity is related to symptoms and somatization in temporomandibular disorders. J Psychosom Res. 2012; 73: 307-12.
20. Bradley LA. Recent approaches to understanding osteoarthritis pain. J Rheumatol. 2004; 70: 54-60.
21. Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci U S A. 2011; 108: 6270-5.
22. Horjales-Araujo E, Demontis D, Lund EK, Vase L, Finnerup NB, Børglum AD, et al. Emotional modulation of muscle pain is associated with polymorphisms in the serotonin transporter gene. Pain. 2013; 154: 1469-76.
23. Venkataramanan V, Gignac M, Dunbar M, Garbuz D, Gollish J, Gross A, et al. The importance of perceived helplessness and emotional health in understanding the relationship among pain, function, and satisfaction following revision knee replacement surgery. Osteoarthr Cartil. 2013; 21: 911-7.
24. Rhudy JL, Williams AE, McCabe KM, Russell JL, Maynard LJ. Emotional control of nociceptive reactions (ECON): Do affective valence and arousal play a role? Pain. 2008; 136: 250-61.
25. Barbosa GAS, Morais MHST. Differential diagnosis between post-polio syndrome symptoms and temporomandibular disorder: clinical case. Braz J Oral Sci. 2013; 12: 57-60.
 Correspondence:
Correspondence:
Maísa Soares Gui
Faculdade de Odontologia de Piracicaba - Unicamp
Av. Limeira, 901 - CP 52
CEP 13414-903 Piracicaba, SP, Brasil.
E-mail: maisa_gui@yahoo.com.br
Received for publication: May 18, 2014
Accepted: August 12, 2014













