Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.13 no.4 Piracicaba Out./Dez. 2014
ORIGINAL ARTICLE
Long-term bond strength, degree of conversion and resistance to degradation of a HEMA-free model adhesive
Fabrício Mezzomo CollaresI; Vicente Castelo Branco LeituneI; Fernando Freitas PortellaI; Fabrício Aulo OgliariII; Susana Maria Werner SamuelI
I Universidade Federal do Rio Grande do Sul – UFRS, School of Dentistry, Dental Materials Laboratory, Porto Alegre, RS, Brazil
II Universidade Federal de Pelotas – UFPEL, Materials Engineering School, Pelotas, RS, Brazil
ABSTRACT
Aim: To evaluate the long-term bond strength, degree of conversion and resistance to degradation in ethanol of HEMA-containing and HEMA-free model adhesive resins of a three-step etch-andrinse adhesive system. Methods: The superficial dentin of 16 bovine incisor teeth was exposed, and the teeth were divided in two groups according to the HEMA concentration in the experimental adhesive (0% and 15%). In each tooth were made 6 cylindrical composite restorations. Half of the tooth restorations were submitted to microshear bond strength test after 24 h and the other half after 6 months. Degree of conversion of experimental resins was determined by Fourier transform infrared spectroscopy. Crosslink density was indirectly determined by the Knoop hardness of five specimens per group before and after immersion in ethanol for 6 h. Results: The group with 0% HEMA showed no difference in bond strength as compared to the group with 15% HEMA after 24 h or 6 months. There was no difference in degree of conversion and crosslink density between groups. Conclusions: HEMA content of the adhesive resin did not influence the bond strength to dentin, degree of conversion or resistance to degradation in ethanol.
Keywords: dental bonding; dentin-bonding agents; light-curing of dental adhesives.
Introduction
The longevity of dental restorations is an important clinical concern1-2. Efficient adhesive resin infiltration and polymerization at the tooth/resin interface are related to the preservation of the results of clinical procedures3. Improvement of the adhesive systems has been associated with the development of different system formulations, as the incorporation of resin monomers with hydrophilic groups increases the bond strength4.
Almost all commercial etch-and-rinse adhesive systems include 2- hydroxyethyl methacrylate (HEMA) or other hydrophilic monomer in their composition5. This hydrophilic monomer is required to enhance infiltration of hydrophobic components into demineralized dentin to promote micromechanical retention of curable monomers. However, the presence of hydrophilic components in the hybrid layer could promote water penetration and degradation of the polymer over time6-8 whereas HEMA increases permeability of the adhesive layer, taking up water and decreasing the mechanical properties of hybrid layer. The influence of HEMA on mechanical properties of polymer structure may be attributed to the low degree of conversion exhibited by polymers containing increased concentration of HEMA9. It is known that a low degree of conversion is related to a low crosslink density10 and decreased mechanical properties of the formed polymer. The bond strength to tooth substrate is directly related to the mechanical properties of the adhesive layer11.
The effect of HEMA on adhesive resin properties has already been examined in a previous study and showed that an increased ratio of HEMA decreases the degree of conversion and ultimate tensile strength, and increases the water sorption and solubility of polymer9. However, more studies are needed to evaluate other properties and the longevity of the adhesive/dentin bond of adhesive resins with or without HEMA, since the presence of a hydrophilic monomer in the adhesive layer could influence the bond strength over time. Hence, the present study tested the null hypothesis that the addition of 15% HEMA in a model adhesive resin will not influence the microshear bond strength, degree of conversion and resistance to chemical degradation.
Material and methods
Materials
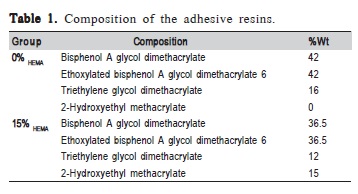
The monomers used were bisphenol A glycol dimethacrylate (BisGMA), ethoxylated bisphenol A glycol dimethacrylate 6 (BisEMA), triethylene glycol dimethacrylate (TEGDMA) and 2-hydroxyethyl methacrylate (HEMA). Two blends with different ratios of HEMA were prepared, one with 0% and another with 15% in weight (Table 1). For each group, 1% mol of camphoroquinone (CQ, Esstech, Essington, PA, USA), used as photosensitizer and 1% mol of N,N-Dimethyl-para-toluidine (DMPT, Fluka, Everett, WA, USA) used as a reducing agent were added to transform the mixtures into light polymerizing blends. The photoactivation for all tests was initiated by a light-emitting diode light source (Radii, SDI, Bayswater, Victoria, Australia), and the irradiance value was confirmed with a digital power meter (Ophir Optronics, North Andover, MA, USA) with 1200mW/cm2.
Microshear bond strength
Sixteen bovine maxillary incisor teeth, stored in 4 °C distilled water for no more than 3 months, were used in this study. The teeth were embedded in acrylic resin and the labial enamel was ground down to expose the superficial dentin. The dentin was ground with 600-grit SiC paper for 30 s in running water12. The teeth were divided in two groups according to the HEMA presence in the model adhesive resins (0% and 15%). The dentin was conditioned for 15 s with 37% phosphoric acid gel and washed for the same time. The water was gently removed with an absorbent paper. A commercial primer composed by water (40-50%), HEMA (35- 45%) and polyalkenoic acid (10-20%) (Primer Scotch Bond Multi Purpose, 3M ESPE, St. Paul, MN, USA) was agitated using disposable applicators on the dentin surface for 10 s and dried for 10 s with an air stream at a distance of 10 cm. Then the model adhesive resins was applied for 5 s using disposable microbrush tips and polymerized for 20 s. In each tooth, 6 cylindrical composite restorations (Z250, 3M ESPE) were made using metallic cylindrical moulds 2 mm high, resulting restorations with 0.88 (± 0.03) mm2 of adhesive area13. Restorations were polymerized for 40 s, and the teeth were stored in 37 °C distilled water. Three of these restorations in each tooth were randomly submitted to a microshear bond strength test after 24 h and the other three after 6 months of storage. The specimens were mounted in a universal testing machine (DL-2000, EMIC, São José dos Campos, SP, Brazil), and shear force was applied at a 1 mm/ min cross-head speed using a steel wire (± 0.4 mm). The wire was positioned in the bond line, and the cylinder was pulled. The bond strengths were expressed in MPa. The failure mode of each specimen was determined under a stereomicroscope at 60x magnification and designated as adhesive, mixed or cohesive failure in either adhesive or resin composite. The means and standard deviations of the groups were analyzed for statistically significant differences by two-way ANOVA for microshear bond strength evaluation. To compare the pattern of failure between groups, the Kruskal-Wallis test was used. Statistical significance was defined as p<0.05.
Degree of conversion
Degree of conversion of the experimental adhesives was evaluated using Fourier transform infrared spectroscopy (FTIR) with a Shimadzu Prestige 21 (Shimadzu, Kyoto, Japan) spectrometer equipped with an attenuated total reflectance device with a horizontal ZnSe crystal and a 45° mirror angle (PIKE Technologies, Madison, WI, USA). A support was coupled to the spectrometer to fix the light curing unit and standardize the distance between the fiber tip and sample at 5 mm. IRSolution software in monitoring scan mode was used, with Happ-Genzel appodization in a range of 1750 to 1550 cm-1 and resolution of 8 cm-1. Analysis was performed at a controlled room temperature of 23±1 °C and 60±1% relative humidity after sample (3 μL) polymerization, which was directly dispensed onto the ZnSe crystal and lightactivated for 20 s., The test was repeated three times (n=3). The degree of conversion was calculated as described in a previous study14, considering the intensity of carbon-carbon double bond stretching vibration (peak height) at 1635 cm- 1 and using the symmetric ring stretching at 1610 cm-1 from the polymerized and unpolymerized samples as an internal standard. The means of the degree of conversion of the groups were compared using the t -test, with p<0.05 indicating statistical significance.
Softening in ethanol
To determine the resistance to degradation, the experimental adhesives were placed in circular elastomeric molds with 4 mm diameter and 2 mm deep, covered with polyester strips and photoactivated for 20 s. Five specimens (n=5) were prepared for each experimental adhesive and then embedded in a acrylic resin with the top in contact with a glass plate and polished in a polisher (Model 3v; Arotec, Cotia, SP, Brazil) with a felt disc embedded with alumina suspension (Alumina 1.0 μm, Arotec) after the specimens were stored at 37 °C for 24 h. The specimens were subjected to a microhardness test in which 9 indentations (15 g/10 s), 100 μm apart from each other and were assessed using a digital microhardness tester (HMV 2, Shimadzu, Tokyo, Japan). The initial Knoop hardness number (KHN1) was registered, and then the specimens were subjected to softening in absolute alcohol for 6 h at 37 °C, when the hardness test was repeated, and the post-conditioning hardness value was measured (KHN2)15. The hardness values between groups were compared by t-test, and the values before and after ethanol immersion were compared by paired t-test, being statistically different if p<0.05.
Results
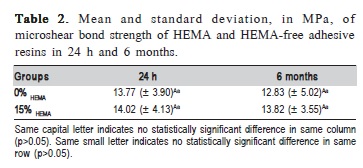
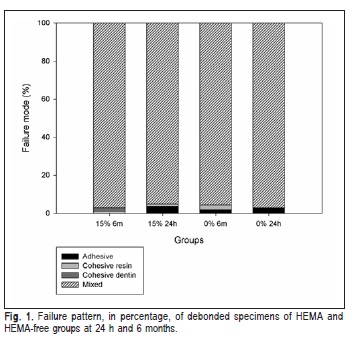
Microshear bond strength values of experimental adhesive with 0 and 15% of HEMA (0% HEMA and 15% HEMA, respectively) to bovine dentin showed no difference between groups (Table 2). The 0% HEMA group showed no statistical difference to the 15% HEMA group at 24 h or 6 months (p>0.05). The fracture mode of almost all specimens was classified as mixed for all groups (Figure 1) and presented no significant difference in failure pattern when correlated with presence of HEMA and storage times.
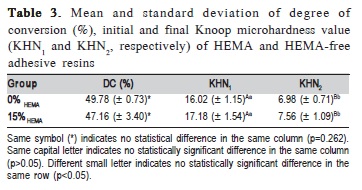
The data for degree of conversion and softening in ethanol are shown in Table 3. For the degree of conversion in 20 s, no significant difference between groups was detected (p=0.262). Initial microhardness evaluation showed no statistical difference among 0% HEMA and 15% HEMA adhesive resins (p=0.211). After 6 h in absolute ethanol, the 15% HEMA resin showed no statistical difference in Knoop microhardness to 0% HEMA adhesive resin (p=0.346). However, 15% HEMA and 0% HEMA adhesive resins showed a statistical reduction in Knoop microhardness values after 6 h in absolute ethanol immersion (p<0.001).
Discussion
Immediate and long-term bond strength at the adhesive/ resin interface influences the efficiency of the resin bond to dentin. Almost all commercial dental adhesive systems contain HEMA in their composition in order to improve wetting of the dentin substrate, promote hydrophobic monomer infiltration and enhance bond strength. This study evaluated the long-term microshear bond strength of experimental adhesive resins with different ratios of HEMA to bovine dentin and showed no statistical difference between groups despite the presence of HEMA or the storage time. Despite the non-significant difference shown for degree of conversion, the Knoop microhardness of both adhesive resins decreased after immersion in ethanol.
There is morphological evidence that hydrophilic adhesive systems behave as semi-permeable membranes16-17. Porous regions in the bonded interface with water-rich and hydrophilic monomer zones could lead to channels for water sorption and leaching of unpolymerized monomers, thus promoting hydrolytic degradation of the polymer. Long-term hybrid layer degradation is explained by the degradation of polymer matrix8 and/or collagen fibrils18 by hydrolysis due to water penetration from the dentin and oral environment through porosities and intermolecular spaces of the polymer network interface with dentin substrate, decreasing the mechanical properties of the polymer formed.
Despite the increase in the percentage of hydrophilic and low molecular weight monomer of the 15% HEMA group, the same adhesive resin compositions showed no difference in ultimate tensile strength between each other, 85.4 and 81.1 MPa for 0% HEMA and 15% HEMA respectively, in a previous study9. However, the water sorption and solubility of 15% HEMA adhesive resin presented significantly higher values than 0% HEMA9. Nevertheless, in the present study, the bond strength showed no difference between the two groups even in a long-term bond strength test. This could be explained by the low viscosity of HEMA, which increases the penetration of adhesive resin into the demineralized dentin of the 15% HEMA group, thus increasing the proportion of hydrophobic monomers in the hybrid layer.
A previous study shows that the microshear bond strength of adhesive resins to bovine dentin did not differ from human dentin19. The same pattern was confirmed in an evaluation using microtensile bond strength test20. Moreover, the scanning electronic microscopy images reveal that bovine and human dentin present similar dentinal morphology after phosphoric acid etching20.
HEMA monomer hydrophilicity contributes to promote bonding to tooth substrate4. Due to its low molecular weight and size, HEMA may easily penetrate demineralized dentin tissue4,21, thus promoting hybrid layer formation. However, increased hybrid layer hydrophilicity could lead to bond interface that is more prone to degradation. In a previous study21, a transmission electronic microscopy evaluation showed the same pattern of spot and cluster-like nanoleakage for a HEMAfree and a HEMA-containing adhesive systems, whereas the HEMA-free adhesives present lower immediate dentin bond strength than the HEMA-containing adhesives. Despite this difference in initial bond strength, a long-term evaluation is still required to confirm the effects of HEMA-containing adhesives' hydrophilicity on the preservation of bonded interface. In this study, no difference between bond strength of 0 and 15% HEMA adhesive resins were found neither immediately nor after 6 months of water storage.
The failure pattern of specimens was almost all mixed for both groups. The microshear bond strength test revealed a nonhomogeneous stress concentration at the dentin substrate22 which could explain these results. However, no significant difference was observed in the failure pattern when correlated with presence of HEMA and storage times. An explanation for these results may be the presence of BisEMA in the composition of the adhesive resins. The BisEMA molecule is similar to BisGMA, with a phenyl central core without the two hydroxyl groups in the backbone, which decreases the viscosity of the comonomer blend23. A decreased viscosity could lead to a higher interpenetration of monomer into the demineralized dentin, proxying the HEMA function in the adhesive resin.
Both adhesive resins evaluated in this study presented a similar degree of conversion. The 0% HEMA showed no difference on softening in ethanol when compared with 15% HEMA. Resistance to degradation after immersion in ethanol is affected by the crosslink density of polymers24. Networks with high crosslink density have reduced solvent uptake due to reduced free space between the polymer chains. Therefore, it is expected that organic solvents would cause less softening in these polymers8. In polymers with a low crosslink density, alcohol can form strong secondary bonds with the polymer chains, penetrate and replace the interchain secondary bonds, and dissolve the material, causing the softening25.
The polymerization behaviors could be affected by increased HEMA content, reducing the degree of conversion, due perhaps to lower monomer reactivity. The 0% HEMA adhesive resin's low polymerization ratio in the initial polymerization seconds9 may result from the high BisGMA content (42% wt). BisGMA has a stiff central core with a hydroxyl group in the backbone that hinders monomer diffusion through the solidifying adhesive and reduces the mobility of unreacted pendant double bonds9. It is known that a high ratio of monofunctional/bifunctional monomers may result in a polymer with low crosslink density, due to its less reactive double bonds26, but this fact was not observed in this study. The crosslink density, indirectly assessed by the softening in ethanol, of the 15% HEMA adhesive resin and of the adhesive resin without monofunctional components showed no significant difference. Nonetheless, the addition of a iodonium salt (e.g. diphenyliodonium hexafluorphosphate) as an alternative photoinitiator could improve the reactivity of methacrylate monomers27, enhancing the degree of conversion27 and improving dentinbond strength28, and thus offsetting the drawbacks of a high viscosity blend, as the 0% HEMA. An in vitro study29 showed similar results for bond strength of HEMA-free experimental adhesive systems compared to a commercial three-step etchand- rinse adhesive system. Additionally, a clinical study showed a high retention rate of non-carious class V restorations after 5 years in function that did not differ from a three-step etch-and-rinse adhesive system containing HEMA30.
The commercial primer (Adper Scotch Bond Multi Purpose, 3M ESPE) used in restorative procedures presents 35-45 wt% of HEMA in its composition, which provide an adequate diffusion of monomers on etched dentin and help to explain the lack of difference in longitudinal microshear bond strength between 0 and 15% HEMA adhesive resins verified in this study. The polymerized HEMA that remained entrapped on the adhesive layer of both tested groups could also make less sensible the detection of long-term bond strength changes promoted by HEMA addition to bonding resin. Even with the significantly higher water sorption rate and solubility of the 15% HEMA adhesive resin used9, a significant degradation of bond strength was not observed. However, the storage period used could be not long enough for a noticeable degradation to occur and differences in bond strength of the tested adhesive resins to be perceived.
Within the limitations of this study, the content of HEMA in the adhesive resin showed no influence on the degradation of bond strength to dentin in the three-step etchand- rinse adhesive system use din this study. It neither influenced the degree of conversion and resistance to degradation of adhesive resin.
Acknowledgements
The author F.F.P. gratefully acknowledges CAPES for the scholarship.
References
1. Demarco FF, Corrêa MB, Cenci MS, Moraes RR, Opdam NJ. Longevity of posterior composite restorations: not only a matter of materials. Dent Mater. 2012; 28: 87-101. [ Links ]
2. Pallesen U, van Dijken JW, Halken J, Hallonsten AL, Höigaard R. Longevity of posterior resin composite restorations in permanent teeth in Public Dental Health Service: a prospective 8 years follow up. J Dent. 2013; 41: 297-306.
3. Peumans M, Kanumilli P, De Munck J, Van Landuyt K, Lambrechts P, Van Meerbeek B. Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dent Mater. 2005; 21: 864-81.
4. Pashley DH, Zhang Y, Agee KA, Rouse CJ, Carvalho RM, Russell CM. Permeability of demineralized dentin to HEMA. Dent Mater. 2000; 16: 7-14.
5. Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007; 28: 3757-85.
6. De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, et al. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005; 84: 118-32.
7. De Munck J, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, Suzuki K, et al. Four-year water degradation of total-etch adhesives bonded to dentin. J Dent Res. 2003; 82: 136-40.
8. Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006; 22: 211-22.
9. Collares Collares FM, Ogliari FA, Zanchi CH, Petzhold CL, Piva E, Samuel SM. Influence of 2-Hydroxyethyl Methacrylate Concentration on Polymer Network of Adhesive Resin. J Adhes Dent. 2011; 13: 125-9.
10. Barszczewska-Rybarek I, Gibas M, Kurkok M. Evaluation of the network parameter in aliphatic poly (urethane dimethacrylate)s by dynamic thermal analysis. Polymer. 2000; 41: 3129-35.
11. Bae JH, Cho BH, Kim JS, Kim MS, Lee IB, Son HH, et al. Adhesive layer properties as a determinant of dentin bond strength. J Biomed Mater Res B: Appl Biomater. 2005; 74: 822-8.
12. Koliniotou-Koumpia E, Kouros P, Koumpia E, Helvatzoglou-Antoniades M. Shear bond strength of a "solvent-free" adhesive versus contemporary adhesive systems. Braz J Oral Sci. 2014; 13: 64-9.
13. Leitune VC, Portella FF, Bohn PV, Collares FM, Samuel SM. Influence of chlorhexidine application on longitudinal adhesive bond strength in deciduous teeth. Braz Oral Res. 2011; 25: 388-92.
14. Collares FM, Portella FF, Leitune VC, Samuel SM. Discrepancies in degree of conversion measurements by FTIR. Braz Oral Res. 2014; 28: 9-15.
15. Leitune VC, Collares FM, Trommer RM, Andrioli DG, Bergmann CP, Samuel SM. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J Dent. 2013; 41: 321-7.
16. Tay FR, Frankenberger R, Krejci I, Bouillaguet S, Pashley DH, Carvalho RM, et al. Single-bottle adhesives behave as permeable membranes after polymerization. In vivo evidence. J Dent. 2004; 32: 611-21.
17. Tay FR, Pashley DH, Yoshiyama M. Two modes of nanoleakage expression in single-step adhesives. J Dent Res. 2002; 81: 472-6.
18. Hebling J, Pashley DH, Tjaderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005; 84: 741-6.
19. Schilke R, Bauss O, Lisson JA, Schuckar M, Geurtsen W. Bovine dentin as a substitute for human dentin in shear bond strength measurements. Am J Dent. 1999; 12: 92-6.
20. Reis AF, Giannini M, Kavaguchi A, Soares CJ, Line SR. Comparison of microtensile bond strength to enamel and dentin of human, bovine, and porcine teeth. J Adhes Dent. 2004; 6: 117-21.
21. Mine A, De Munck J, Van Landuyt KL, Poitevin A, Kuboki T, Yoshida Y, et al. Bonding effectiveness and interfacial characterization of a HEMA/ TEGDMA-free three-step etch&rinse adhesive. J Dent. 2008; 36: 767-73.
22. Placido E, Meira JB, Lima RG, Muench A, de Souza RM, Ballester RY. Shear versus micro-shear bond strength test: a finite element stress analysis. Dent Mater. 2007; 23: 1086-92.
23. Ogliari FA, Ely C, Zanchi CH, Fortes CB, Samuel SM, Demarco FF, et al. Influence of chain extender length of aromatic dimethacrylates on polymer network development. Dent Mater. 2008; 24: 165-71.
24. Schneider LF, Moraes RR, Cavalcante LM, Sinhoreti MA, Correr-Sobrinho L,Consani S. Cross-link density evaluation through softening tests: effect of ethanol concentration. Dent Mater. 2008; 24: 199-203.
25. Soh MS, Yap AU. Influence of curing modes on crosslink density in polymer structures. J Dent. 2004; 32: 321-6.
26. Rueggeberg F, Tamareselvy K. Resin cure determination by polymerization shrinkage. Dent Mater. 1995; 11: 265-8.
27. Gonçalves LS, Moraes RR, Ogliari FA, Boaro L, Braga RR, Consani S. Improved polymerization efficiency of methacrylate-based cements containing an iodonium salt. Dent Mater. 2013; 29: 1251-5.
28. Leal FB, Lima GS, Collares FM, Samuel SM, Petzhold CL, Piva E, et al.Iodonium salt improves the dentin bonding performance in an experimental dental adhesive resin. Int J Adhesion and Adhesives. 2012; 38: 1-4.
29. Zanchi CH, Münchow EA, Ogliari FA, Chersoni S, Prati C, Demarco FF et al. Development of experimental HEMA-free three-step adhesive system. J Dent. 2010; 38: 503-8.
30. Van Landuyt KL, De Munck J, Ermis RB, Peumans M, Van Meerbeek B. Five-year clinical performance of a HEMA-free one-step self-etch adhesive in noncarious cervical lesions. Clin Oral Investig. 2014; 18: 1045-52.
 Correspondence:
Correspondence:
Fabrício Mezzomo Collares
Faculdade de Odontologia - Laboratório de
Materiais Dentários
Universidade Federal do Rio Grande do Sul
Rua Ramiro Barcelos 2492
CEP: 90035-003 Porto Alegre, RS, Brasil
E-mail: fabricio.collare@ufrgs.br
Received for publication: August 14, 2014
Accepted: November 25, 2014













