Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Brazilian Journal of Oral Sciences
versão On-line ISSN 1677-3225
Braz. J. Oral Sci. vol.14 no.4 Piracicaba Out./Dez. 2015
ORIGINAL ARTICLE
Evaluation of hemosponge in promoting dental socket healing after 3rd mandibular premolar extraction in a feline model
Azin TavakoliI; Alireza SagartI
I Islamic Azad University, Faculty of Veterinary Medicine, Garmsar branch, Gamsar, Iran
ABSTRACT
Aim: To investigate the healing process following use of collagen sponges in the dental socket after extraction. Wound complications during the study were also evaluated. Methods: 32 cats were included in this study. IV administration of the combination of diazepam (0.22 mg/kg) and ketamine (10 mg/kg) was used to induce general anesthesia. Surgical extraction of both 3rd mandibular premolars was performed. The open dental sockets were divided in two groups. In Group A, the open dental socket on the left side was closed using 4-0 Monocryl in simple interrupted pattern. In Group B, the right dental socket was filled with lyophilized hydrolyzed collagen and the buccal and lingual flaps were sutured using 4-0 Monocryl and simple interrupted pattern. Meloxicam (0.2 mg/ kg) was used to manage the post-extraction pain in all cats. Ampicilline 20 mg/kg was used as prophylaxis. The wounds were observed during the study to evaluate any signs of inflammation or dehiscence. Radiographs were taken to compare healing of the socket 3 weeks after the procedure. A 1 mm biopsy punch sample was taken from sockets in all cats for comparison of the healing in both groups. Results: Hemorrhage occurred only in the sockets of Group A. Remission of radiolucent area occurred in both groups. Mean score of inflammation was lower and mean scores of fibrotic reaction and fibroplasia were higher in Group B (p<0.05). Conclusions: Use of hemosponge in alveolar socket may accelerate fibroplasia and formation of the connective tissue and reduce inflammation after tooth extraction. Therefore, post-extraction use of the hemostatic agent in the dental socket is recommended.
Keywords: tooth socket; tooth extraction; wound healing; cats.
Introduction
Dental extraction is a very frequent procedure performed both in human and veterinary dentistry. Following dental extraction complications may occur leading to discomfort for the patient. The most frequent complications occurring after dental extraction include hemorrhage from the socket, swelling and pain. More serious complications are infection of the socket, alveolar osteitis and osteomyelitis1-3. Both anatomical and physiological changes in the socket after exodontia, which are generally defined as alveolar bone resorption, affect adversely the following implant therapy as well4-6. Different techniques are used to manage and preserve the alveolar socket after extraction. Simple closure with sutures and soft tissue coverage of the wound prevents the accumulation of debris and fluid in the socket. Moreover, topical applications of antibiotics or anti-inflammatory medications and different absorbable or non-absorbable biomaterials have been suggested to be used in the dental socket7-8. Sponges made of collagen, the oldest known protein, are widely used for preservation of dental socket. Several studies have been performed to assess the effect of collagen sponges in the healing of dental sockets9-11. Reports indicated that the collagen sponges are advantageous and accelerate healing of the wound by stabilizing the blood clot and protecting the alveolar lining of the socket8. There is no report available about the histopathological changes in the socket when collagen sponges are used. Therefore, the aim of this study was to compare dental socket healing with and without topical application of collagen sponges after 3rd mandibular premolar extraction in a feline model.
Material and methods
The study started after the ethical approval was received from the Research and Ethical Committee of the University (# 5238), according to the National regulations in researches on indigenous animals. Thirty-two adult domesticated short hair cats were included in the study.
Food was restricted 4 to 6 h prior to surgery in the animals. IM acepromazine (0.05 mg/kg, Neurotranq®, Alfasan, Woerden, The Netherlands) was used as premedication and general anesthesia was induced by IV administration of diazepam (0.22 mg/kg Zepadic®; Caspian Tamin Pharmaceutical Co., Rasht, Iran) and ketamine (10 mg/kg Ketalar®; Alfasan) combination. Cleansing of the mouth with 0.2% chlorhexidine solution was performed to reduce oral contamination prior to the procedure. The incision was made with a scalpel blade #11 on the buccal and lingual gingival margin of the right and left 3rd mandibular premolar teeth in all cats. A dental elevator was introduced into the periodontal space to stretch the periodontal ligament. Finally, dental forceps was used and the tooth extracted. Following extraction, the sockets were divided into two groups. In Group A, the dental socket on the left side was closed using 4-0 monocryl in simple interrupted pattern. The right dental socket was filled with lyophilized hydrolyzed collagen (Hemospon; Technew, Rio de Janeiro, RJ, Brazil) and in Group B, the buccal and lingual flaps were sutured using 4-0 Monocryl with simple interrupted suture pattern. Intravenous injection of meloxicam (0.2 mg/kg Metacam®; Boehringer-Ingelheim, Ingelheim, Germany) was used to manage the post-extraction pain in all cats. Intramuscular administration of ampicilline 20 mg/kg was used as prophylaxis. The wounds were observed daily in the first week and then weekly during the study to evaluate any signs of inflammation or dehiscence. Radiographs were taken to compare healing of the dental socket 3 weeks after the procedure in both groups. In all cats, a biopsy sample was taken from the dental socket using a 1 mm biopsy punch for histopathological evaluation 3 weeks following the procedure. Sixty-four dental sockets were evaluated in the present study.
The tissue was fixed in 10% buffered formalin and processed in a tissue processor. Paraffin-embedded tissue sections were stained by Harris hematoxylin and eosin (H&E) method. The stained sections were viewed under light microscope at 4X and 10X magnifications for examination. A single veterinary pathologist viewed the slides and graded them according to epithelialization, fibrotic reaction, fibroplasia, inflammation and edema. Epithelialization was graded as non-existent (1), start of migration (2), covering less than half the wound surface (3), covering more than half the wound surface (4), irregular covering of the wound (5) and normal covering of the wound (6). Inflammation was graded as infiltration of acute inflammatory cells in wound clot and perivascular regions more than 1.2 of high power field (HPF) (1), between 1.2-1.4 of HPF (2), less than 1.4 of HPF (3), infiltration of acute inflammatory cells in the clot, perivascular regions and in connective tissue (4), infiltration of acute inflammatory cells in wound clot and perivascular regions (5), infiltration of acute inflammatory cells just in wound clot (6), infiltration of acute inflammatory cells in wound margin (7) and no inflammation(8). Fibrotic reaction graded as no deposition of collagen (1), new collagen bundle deposition (2), lamina propria fibrosis (3), dermal fibrosis (4) and fibrosis in all layers (5). Fibroplasias graded as no granulation tissue (1), loose granulation tissue (2), cell-rich granulation tissue (3), a few collagen fiber depositions (4), more collagen deposition (5) and remodeling of collagen bundles (6).
Means of the measured variables were compared between the groups using t-test. p values less than 0.05 were considered statistically significant.
Results
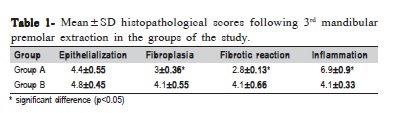
Hemorrhage was reported only in the sockets of Group A. Wound dehiscence occurred in 9 sockets and infection was observed in 2 sockets in the same subject, mostly in Group A following the first week after the procedure. Radiographs revealed remission of radiolucent areas in both groups. However, soft tissue swelling was noted in radiographs of 9 patients in Group A and 4 patients in Group B, respectively. Histopathological studies indicated that the mean scores of fibroplasia and fibrotic reaction in Group B were statistically higher in comparison with Group A (p 0.05) (Figures 1 and 2). The inflammation scores of wound edges were also significantly lower in Group B than in Group A (p<0.05). Epithelialization scores were not statistically different between the groups (p>0.05) (Table 1).
Clinical evaluation of the wounds was performed during the study. The observed complications are listed in Table 2.
Discussion
The purpose of the present study was to investigate the healing process following the use of collagen sponges in the dental socket after extraction. It was also evaluate the efficacy of collagen sponges by assessing the rate of wound complications during the study. The healing of 64 dental sockets after 3rd mandibular premolar extraction was evaluated in the present study. Wound complication rate was higher in dental sockets that were routinely closed without the hemosponge. Hemorrhage after closure was evident in 11 patients and occurred only in the sockets of Group A, which highlights the nature of hemosponge as a hemostatic device. Wound dehiscence occurred in 8 and 1 of the subjects in Group A and B, respectively, probably due to the inflammation that caused discomfort on the left side.

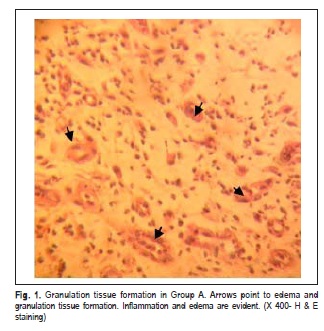
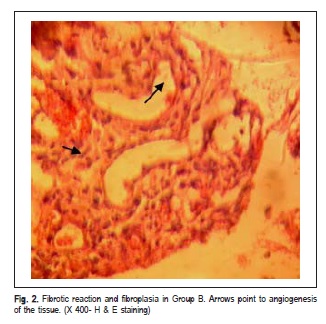
Bleeding occurs immediately after dental extraction and inflammation, which is the first phase of wound repair that takes place within a few minutes after exodontia. Clot fills the remaining socket. The clot acts as a lining to the socket, which preserves the alveolar bone and exposed nerve ends12-13. Fibroplasia continues and the clot is replaced by granulation tissue usually a week after the procedure. Then the epithelial cells recognize the granulation tissue as a connective tissue and start to migrate and cover the newly formed granulation tissue. Remodeling begins in the underneath granulation tissue to form the provisional matrix. Finally gradual mineralization of the matrix occurs to form the lamellar bone14-15. Therefore, clot formation works as a beginning stage of the socket-healing cascade. Then it works as a matrix for angiogenesis and fibroplasia. If the clot is washed away or fibrinolysis happens, early swelling and sever pain will occur. Thus, the more stable the clot in the dental socket, the less inflammation, alveolar osteitis and pain will be expected16-17.
Hemosponge is a soft, white, sterile and hemostatic spongelike structure made of lyophilized, hydrolyzed collagen of porcine origin, which is capable of absorbing and holding blood many times its weight. The sponge is completely absorbed usually within 4 to 6 weeks18. The risk of postextraction bleeding was reported to decrease when the collagen hemostatic sponge is used in the extraction socket19.
Elimination of the radiolucent area of the dental socket was observed in both groups, which underscores that the healing process continued without complication in most sockets. Histopathological evaluation of the wounds 3 weeks after the extraction showed that the score of inflammation was significantly less when the hemosponge was used as in Group B in comparison to simple closure, as in Group A. Considering the score of fibroplasia and fibrotic reaction, the best results were obtained in Group B. As mentioned above, the maintenance of blood clot acts as an inflammation preventive factor during socket healing. Therefore, efforts were made to stabilize the clot at the early stages of the healing. Hemostatic sponges not only preserve the clot and work as hemostatic agent, but also prevent the collapse of newly formed soft tissue into the socket. This extracellular matrix establishes a scaffold so that fibroplasia and formation of the granulation tissue is accelerated. Thus, bone healing in the socket is stimulated. Jenkins20 suggested that the spongy structure of the gelatin sponge is initially responsible for the stimulation of clot formation, and that the structure causes damage to the platelets in bleeding. This means that releasing of prothrombin and calcium in the clot could be sufficient to start the clotting mechanism. In the present study, fewer complications were observed in the sockets treated with hemosponge, which is consistent with previous studies. Choo et al.8 reported less rates of complication in patients with 3rd molar extraction by the use of absorbable collagen sponges.
The scores of epithelialization were not significantly different between the groups. Margo21studied the healing process of the dental socket in a rat model using microfibril collagen hemostat. He reported that the device interferes with the early stage of healing of the socket, but it does not affect the final result of the healing. This finding is in agreement with our findings. Although the hemosponge did not facilitate the epithelialization, occurrence of wound dehiscence and inflammation was not increased. We have limited access to information about the animals after discharges, so that a longterm follow up of the subjects was not undertaken. Therefore, clinical trials and long-term prospective studies of the use of hemosponge are advised. Post-operative computed tomography scans of the dental sockets were not available. If available they may provide more detailed and precise information about the healing and bone formation of the dental socket. Infection of the socket occurred in both sockets of the same patient. Oral hygiene of the patient seems important for reduction of the infection risk in the socket after extraction. Therefore, post- extraction wound care is highly recommended specially during the first week after dental removing.
Hemosponge is a non-toxic and inexpensive structure that provides hemostasis, stabilizes clot, promotes healing and ridge preservation of the socket22-23. In addition, less inflammation of the related soft tissues causes less pain and discomfort for the patient following extraction.
Based on clinical, radiological and histological results of the present study, the use of hemosponge in the alveolar sockets may accelerate fibroplasia and formation of the connective tissue and reduces inflammation of the socket after extraction of the 3rd mandibular premolar teeth. Therefore, post-extraction use of the hemosponge in the dental socket is recommended.
References
1. Bouloux GF, Steed MB, Perciaccante VJ. Complications of third molar surgery. Oral Maxillofac Surg Clin North Am. 2007; 19: 117-28. [ Links ]
2. Bui CH, Seldin EB, Dodson TB. Types, frequencies, and risk factors for complications after third molar extraction. J Oral Maxillofac Surg. 2003; 61: 1379-89.
3. Hwang JK, Kim KW. Complications of impacted third molar extraction: retrospective study. J Korean Assoc Oral Maxillofac Surg. 2010; 36:119-24.
4. Pietrokovski J, Massler M. Ridge remodeling after tooth extraction in rats. J Dent Res. 1967; 46: 222-31.
5. Boyne PJ. Osseous repair of the postextraction alveolus in man. Oral Surg Oral Med Oral Pathol. 1966; 21: 805-13.
6. Devlin H, Sloan P. Early bone healing events in the human extraction socket. Int J Oral Maxillofac Surg. 2002, 31: 641-5.
7. Alexander RE. Dental extraction wound management: a case against medicating postextraction sockets. J Oral Maxillofac Surg. 2000; 58: 538-55.
8. Choo H, Jung HD, Kim BJ, Kim CH, Jung YS. Complication rates in patients using absorbable collagen sponges in third molar extraction sockets: a retrospective study. J Korean Assoc Oral Maxillofac Surg. 2015; 41: 26-9.
9. Oliveira MR, Martins EG, Mariano RC, Sonoda CK, Garcia R, Morais W. Tissue engineering: using collagen Type I matrix for bone healing of bone defects.
10. J Craniofac Surg. 2013; 24:e394-6.
11. Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm. 2001; 221:1-22.
12. Rao KP. Recent developments of collagen-based materials for medical applications and drug delivery systems. J Biomater Sci Polym Ed. 1995; 7: 623-45.
13. Amler MH. The time sequence of tissue regeneration in human extraction wounds. Oral Surg Oral Med Oral Pathol.1969; 3: 309-18.
14. Clafin RS. Healing of disturbed and undisturbed extraction wounds. J Am Dent Assoc. 1996; 23: 945-59.
15. Pagni G, Pellegrini G, Giannobile WV, Rasperini G. Postextraction alveolar ridge preservation: biological basis and treatments. Int J Dent. 2012; 2012: 1-13
16. Liu J. Mechanism of guided bone regeneration: a review. Open Dent J. 2014; 8: 56-65.
17. Nilsson BG. Fibrinolytic activity in alveoli after tooth extraction. Odontology, 1968, 19: 197-204.
18. Vezeau PJ. Dental extraction wound management: medicating postextraction sockets. J Oral Maxillofac Surg. 2000; 58: 531-7.
19. Ogle OE. Perioperative hemorrhage. In: Dym H, Ogle OE. Atlas of minor oral surgery. Philadelphia, PA: Saunders; 2000. p.62-3.
20. Svensson R, Hallmer F, Englesson CS, Svensson PJ, Becktor JP. Treatment with local hemostatic agents and primary closure after tooth extraction in warfarin treated patients. Swed Dent J. 2013; 37: 71-7.
21. Jenkins HP, Janda R. Studies on the use of gelatin sponge or foam as a hemostatic agent in experimental liver resections and injuries to large veins. Ann Surg. 1946; 124: 952-61.
22. Magro FO, Garcia R, Magro FN. Histologic study of use of microfibrillar collagen hemostat in rat dental sockets. Braz Dent J. 2003; 14: 12-7.
23. Mahesh L, Kurtzman G, Shukia S. Regeneration in Periodontics: Collagen - A Review of Its Properties and Applications in Dentistry. Compend Contin Educ Dent. 2015; 36: 358-63.
24. Park BJ. Ridge preservation following tooth extraction using an absorbable gelatin sponge. OHDM. 2015; 14: 271-3.
 Correspondence:
Correspondence:
Azin Tavakoli
PO Box: 3581631167
Garmsar, Iran
E-mail: azin.tavakoli@gmail.com
Received for publication: November 16, 2015
Accepted: December 22, 2015













