Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Odontologia Clínico-Científica (Online)
versão On-line ISSN 1677-3888
Odontol. Clín.-Cient. (Online) vol.11 no.1 Recife Jan./Mar. 2012
ARTIGO ORIGINAL / ORIGINAL ARTICLE
Mast cell in dental pulp: does it have a role?
Mastócitos na polpa dental: eles têm uma função?
Celso Martins Queiroz-JúniorI; Cinthia Mara da Fonseca PachecoII; Sara Vieira MarçalIII; Poliana Cristina Costa de MeloIII; Kátia Lucy de Melo MaltosIII
IPhD student, Department of Morphology, Institute of Biological Sciences, Universidade Federal de Minas Gerais.
IISc.D, PhD, Faculty of Health and Biological Sciences, Centro Universitário Newton Paiva
IIIBSc, Department of Restorative Dentistry, Dental School, Universidade Federal de Minas Gerais.
RESUMO
Os mastócitos desempenham um importante papel em uma variedade de processos biológicos e participam, ativamente, da resposta inflamatória. Existe, no entanto, uma controvérsia na literatura a respeito da presença de mastócitos em polpas dentais. O presente trabalho procurou responder essa controvérsia no que se refere à presença de mastócitos no tecido pulpar de ratos e humanos em condições normais e durante a inflamação. Para isso, polpas inflamadas e não inflamadas de humanos e ratos foram coletadas e analisadas, utilizando-se a técnica histoquímica do azul de toluidina e a técnica de imunohistoquímica. Nossos resultados mostraram a ausência de mastócitos em polpas dentais de ratos e humanos tanto em condições normais quanto durante a inflamação. O papel dos mastócitos na resposta inflamatória da polpa dental não é claro. Fatores de crescimento e citocinas envolvidas na sua migração, desenvolvimento e sobrevivência podem estar ausentes no tecido pulpar e necessitam de futuras investigações.
Descritores: Polpa dentária; Mastócitos; Inflamação; Tecido do Conjuntivo.
ABSTRACT
Mast cells play an important role in a variety of biological processes and actively participate in the inflammatory response. There is a controversy in the literature whether mast cells are present in dental pulp. In this investigation we sought to answer the question concerning the presence of mast cells in human and rat dental pulp tissues, under normal and inflammatory conditions. Human and rat dental pulp under normal and inflammatory conditions were analyzed using toluidine blue histochemistry and immunohistochemistry techniques. Our results showed that inflamed and non-inflamed dental pulps neither from humans nor from rats presented mast cells. The role of mast cells in the inflammatory dental pulp response is not clear. Growth factors and cytokines involved in their migration, development and survival could be absent in this tissue and need further investigations.
Descriptors: Dental pulp; Mast Cell; Inflammation; Conective Fissue.
INTRODUCTION
It has been a matter of controversy in the literature whether mast cells are present in dental pulp. Studies using human samples have shown that such cells are present in dental pulps1,2 whilst others have demonstrated the absence of those cells3,4,5. In addition there is a lack of information regarding the presence of mast cells in dental pulp of rats, which are animals used to study important aspects of the pathophysiology of dental pulp in vivo6.
Mast cells play an important role in a variety of biological processes including allergic reactions, atherosclerosis and inflammation7,8. The activation of these cells leads to the release of chemical mediators such as histamine and arachidonic acid metabolites, which increase vascular permeability and tissue swelling9.
On the other hand, dental pulp is a connective tissue surrounded by hard walls of mineralized tissue. Such characteristic decreases its possibilities of swelling in response to injuries4,10.
Since mast cells are active during inflammatory responses it becomes crucial to find out if these cells are actually present and play a role in dental pulp under normal and inflammatory conditions either in humans or in rats.
Therefore, the purpose of the present work was, under the same experimental conditions, to identify mast cells in healthy and inflamed dental pulp from rats and humans using toluidine blue histochemistry and immunohistochemistry techniques.
MATERIAL AND METHODS
Rat Tissue Samples
Male Holtzman rats (260-320g) were anesthetized (ketamine-xylazine 90-15mg/kg, i.m.); dental pulp from the upper first molar (n=6) was exposed and inflammation was locally induced by bacterial lipopolysaccharide (LPS, Escherichia coli; batch B111; 1.2μg/site, 1μl; Sigma Chemicals, St. Louis, MO, USA). The pulp cavity was sealed with glass ionomer (Vidrion®, SSWhite, São Paulo, SP, Brazil) and the animals were killed by cervical dislocation 6h after LPS stimuli, as previously described6. The maxillae halves were excised and processed for histopathological analysis. Molars teeth (n=6), with no pulp exposure, were used as non-inflamed controls, and the gingival tissue (n=12) surrounding the teeth was used as a positive control for mast cell detection. All experiments were conducted in accordance with the ethical guidelines of the Institutional Ethical Committee.
Human Tissue Samples
Human pulp tissues were obtained from teeth of patients attending Dental Surgery, Orthodontic and Endodontic clinics of Universidade Federal de Minas Gerais Dental School. Following diagnosis and indication for tooth extraction, patients that signed the informed consent were enrolled in the study. This study was conducted according to the ethical guidelines of the Institutional Ethical Committee, which approved the protocols described throughout the text (Protocol Number 497/07).
Inflamed human pulp samples (n=12) were obtained from teeth with clinical signs and symptoms of acute pulpitis and with clinical dentine caries, but with no exposed pulp. Samples were immediately immersed in 10% formaldehyde for 48h and then demineralized in 10% ethylenediaminetetraacetic acid (EDTA, Synth, Diadema, SP, Brazil) solution (pH 6.0) for 4 months. The specimens were dehydrated in ethanol, immersed in xylene and embedded in paraffin for further analysis. Healthy pulps (n=5) were obtained from third molars and pre-molar extracted for orthodontic reasons. These pulps were used as non-inflamed controls and gingival tissue (n=2) obtained from periodontal surgery was used as a positive control for mast cells.
Both rat and human tissue samples were stained with hematoxilin and eosin (H/E), toluidine blue or immunohistochemistry for tryptase.
Toluidine Blue (TB) Histochemistry
TB method was modified from Heaney et al.11. Briefly, sections (4μm) were deparaffinized in xylene and hydrated with water. TB staining was performed with a 1% TB solution (Synth, Diadema, SP, Brazil) diluted in phosphate buffer (pH 5,7) for 120s. Soon afterward, sections were quickly dehydrated through 96% ethanol and p.a. acetone, after rinsing in phosphate buffer for 1min. They were then immersed in xylene and mounted in synthetic resin.
Immunohistochemistry (IH)
Paraffin-embedded tissues were sectioned (4μm) and collected in serial sections on glass slides coated with 2% 3-aminopropyltriethylsilane (Sigma Chemicals, St. Louis, MO, USA) for processing by standard IH technique (immunoperoxidase: avidin-biotin-peroxidase). Samples were deparaffinized by immersion in xylene, followed by ethanol and then immersion in citrate buffer (pH 6.0; Sigma-Aldrich Co., St Louis, MO, USA) for 20min at 95°C for antigen retrieval, except for sections that would be examined with the mouse anti-human mast cell tryptase monoclonal antibody (M7052). Soon afterward, sections were incubated with 3% hydrogen peroxide diluted in Tris-buffered saline (TBS; pH 7.4) for 30min. Thereafter, sections were incubated at 4°C overnight in a humidified chamber with one of the following primary antibodies: rabbit polyclonal anti-rat mast cell tryptase (clone FL-275, Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted at 1:2 000 and mouse monoclonal anti-human mast cell tryptase (clone M7052, Dako, Glostrup, Denmark), diluted at 1:2 000. All antibodies were diluted in 1% PBS bovine serum albumin. Following the incubation with the primary antibodies, sections were washed in TBS with Triton X-100 p.a. (H282, Mallinckrodt, Phillipsburg, NJ, USA) and treated with the labeled streptavidin-biotin kit (K0492, Dako). Samples were then incubated in a 3,3μ-diaminobenzidine (DAB) chromogen solution (K3468, Dako) for 3min at room temperature. Finally, after washing with distilled water, the slides were counterstained with Mayer's hematoxylin and were covered. Negative controls consisted of sections in which primary antibodies were omitted and replaced with non-immune rabbit (X0910, Dako) or mouse (X0910,
Dako) serum.
RESULTS
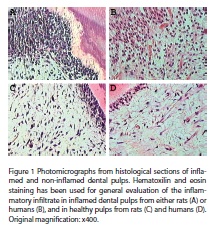
The presence and role of mast cells in many tissues under different conditions, such as inflammation, have been consistently unraveled. Nevertheless their recruitment to dental pulp remains an open question. The present study aimed to answer such question. Firstly, analysis of hematoxilin/eosin stained slides of inflamed dental pulps from either humans or rats showed a mixed polymorphomononuclear inflammatory infiltrate, with a predominance of neutrophils.
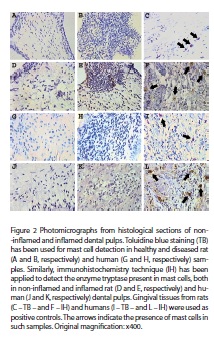
As can be observed in figura 1, TB histochemistry did not identify mast cells in non-inflamed and inflamed pulps from either rats (A, B) or humans (G, H); nevertheless, in the same experimental conditions this technique stained mast cells in rat and human gingival tissues (C, I). Likewise, immunohistochemistry for tryptase did not identify mast cells in dental pulps from rats and humans either under normal or inflamed conditions (D, E, J, K).
DISCUSSION
Although mast cells have been firstly identified more than a century ago12 much still remains to be understood about their development and recruitment to a tissue. Mast cells are derived from pluripotential hematopoietic stem cells and they leave the bone marrow as progenitors. Once in the blood stream they migrate to different tissues where they complete their maturation process13,14. Local factors present in the tissue will be responsible for progenitor's final development15.
Our results did not show mast cells in dental pulps either from humans or rats under inflamed and non-inflamed conditions what implies that such cells are not important during dental pulp inflammation as they are in other tissues16,17. It is well known that mast cells are notable modulators of the inflammatory response16,17 as can be observed by their increased number in inflamed tissues18. On the other hand, dental pulp inflammation is a more complex process than that found in tissues freely able to swell. It involves both nervous and vascular reactions, which are key components of neurogenic inflammation10.
As it has been widely studied, neuropeptides such as substance P (SP) and calcitonin gene-related peptide (CGRP) play an active role during neurogenic pulp inflammation, controlling its blood flow and regulating different stages of inflammation and tissue repair19. But it is also known that the neuropeptide SP interacts mainly with mast cells in the tissue to induce the release of histamine and thereby cause elevation of vascular permeability and local blood pressure to solve the microbe challenge20. In this regard, the absence of mast cells in dental pulp as pointed out herein could explain the poor results previously found when SP was used to induce vascular permeability in rat dental pulp6, and also could have a positive role in this environment, not allowing an enormous swelling which could lead to a quickly necrosis of pulp tissue. Our findings corroborate those from a recent work using IH in human samples, which only identified mast cells in pulp polyps, a specific condition in which dental pulp is able to swell; but the same study did not find mast cells in encased pulp tissues even when they were inflamed21. Although this lack of mast cells in dental pulp could be a positive characteristic to this tissue, such absence could also interfere with the defense mechanisms of dental pulp since mast cells have recently gained new importance as immunoregulatory cells and as a source of cytokines and chemokines17,22.
The mechanisms underlying the absence of mast cells in dental pulp are still a matter of speculation. It is known that Stem Cell Factor (SCF) and its receptor c-kit are essential for mast cell development and growth in a tissue23. A recent work has identified both SCF and c-kit in cultures of cells derived from human dental pulp24. However, SCF-mediated mast cell development is regulated by other factors including cytokines like IL-3, IL-9, and IL-10 and growth factors like nerve growth factor (NGF)25. These cytokines work directly stimulating proliferation of uncommitted progenitors or as cofactor for mast cell proliferation26,27. NGF stimulates the differentiation and proliferation of mouse bone marrow derived mast cells and it works synergistically with SCF suppressing mast cell apoptosis in humans28.
Since SCF and c-kit seems to be present in dental pulp some of these other factors, which are crucial for final mast cell development and which work modulating SCF activity, might be lacking in dental pulp. Conversely, this tissue may harbor important inhibitory factors for mast cell development and/or survival such as the granulocyte-macrophage colony-stimulating factor (GM-CSF), a well-known inhibitor of mast cell development in both rodents29 and human systems30.
CONCLUSION
In conclusion our results demonstrated, through different standard techniques for mast cells detection, that dental pulps from either humans or rats did not present such cells. Regarding the absence of those cells in rat pulps such finding contributes to validate the in vivo studies using those animals, especially those in which inflammation is induced in pulp tissues. The mechanisms involved in explaining the absence of mast cells in dental pulp are still a matter of speculation. Thus, further studies are required to investigate the growth factors, cytokines and other mediators involved in mast cell chemotaxis, maturation and survival that could be absent in dental pulp.
ACKNOWLEDGMENTS
The authors are grateful to T.A. Silva for her helpful assistance. This work was supported by the Pró-Reitoria de Pesquisa from Universidade Federal de Minas Gerais (PRPq-UFMG), FAPEMIG and CNPq.
REFERENCES
1. Walsh LJ, Davis MF, Xu LJ, Savage NW. Relationship between mast cell degranulation and inflammation in oral cavity. J Oral Pathol Med. 1995; 24(6): 266-72. [ Links ]
2. Walsh LJ. Mast cells and oral inflammation. Crit Rev Oral Biol Med. 2003; 14(3): 188-98. [ Links ]
3. Kerezoudis NP, Olgart L, Edwall L. Evans blue extravasation in rat dental pulp and oral tissues induced by electrical stimulation of the inferior alveolar nerve. Arch Oral Biol. 1993; 38(10): 893-901. [ Links ]
4. Jontell M, Okiji T, Dalhgren N, Bergenholtz G. Immune defense mechanisms of the dental pulp. Crit Rev Oral Biol Med. 1998; 9(2): 179-200. [ Links ]
5. Olgart LM, Kerezoudis NP. Nerve pulp interactions. Arch Oral Biol. 1994; 39(Suppl) 47-54. [ Links ]
6. Maltos KLM, Menezes GB, Caliari MV, Rocha OA, Santos JMM, Alves DLF, et al. Vascular and cellular responses to pro-inflammatory stimuli in rat dental pulp. Arch Oral Biol. 2004; 49(6): 443-50. [ Links ]
7. Castells M. Mast cell mediators in allergic inflammation and mastocytosis. Immunol Allergy Clin North Am. 2006; 26(3):465-85. [ Links ]
8. Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004; 4(10): 787–99.
9. Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000; 173: 131-40. [ Links ]
10. Kim S. Neurovascular interactions in the dental pulp in health and inflammation. J Endodont. 1990; 16(2): 48-53. [ Links ]
11. Heaney LG, Leggett P, Maxwell P, Bharucha H, Ennis M. A comparison of three standard methods of identifying mast cells in endobronchial biopsies in normal and asthmatic subjects. Allergy. 1997; 52(8): 836–43.
12. Kitamura Y, Ito A. Mast cell-committed progenitors. Proc Natl Acad Sci USA. 2005; 102: 11129-30. [ Links ]
13. Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978; 52(2): 447–52.
14. Kitamura Y, Go S. Decreased production of mast cells in S1/S1d anemic mice. Blood. 1979; 53(3): 492–97.
15. Kirshenbaum AS, Goff JP, Kessler SW, Mican JM, Zsebo KM, Metcalfe DD. Effect of IL-3 and stem cell factor on the appearance of human basophils and mast cells from CD34+ pluripotent progenitor cells. J Immunol. 1992; 148(3): 772–7.
16. Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997; 77(4): 1033-1079. [ Links ]
17. Galli SJ, Maurer M, Lantz CS. Mast cells as sentinels of innate immunity. Curr Opin Immunol. 1999; 11(1): 53-59. [ Links ]
18. Viegas M, Gomez E, Brooks J, Davies RJ. Changes in nasal mast cell numbers in and out of the pollen season. Int Arch Allergy Appl Immunol 1987; 82(3-4): 275-276. [ Links ]
19. Olgart L. Neural control of pulpal blood flow. Crit Rev Oral Biol Med. 1996; 7(2): 159-71. [ Links ]
20. Hargreaves KM, Swift JQ, Roszkowski MT, Bowles W, Garry MG, Jackson DL. Pharmacology of peripheral neuropeptides and inflammatory mediator release. Oral Surg Oral Med Oral Pathol. 1994; 78(4): 503-10. [ Links ]
21. Freitas P, Novaretti CP, Rodini CO, Batista AC, Lara VS. Mast cells and lymphocyte subsets in dental pulps from healthy and carious human teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103(5): e-95-e102. [ Links ]
22. Jamur MC, Grodzki ACG, Berenstein EH, Hamawy, Majed MH, Siraganian RP, et al. Identification and characterization of undifferentiated mast cells in mouse bone marrow. Blood. 2005; 105(11): 4282-9. [ Links ]
23. Chabot B, Stephenson BA, Chapman VM, Besmer P, Bernstein A. The protooncogene c-kit enconding a transmembrane tyrosine kynase receptor maps to the mouse W locus. Nature. 1988; 335(6185): 88-9. [ Links ]
24. Gagari E, Rand MK, Tayari L, Vastardis H, Sharma P, Hauschka PV, et al. Expression of stem cell factor and its receptor, c-kit, in human oral mesenchymal cells. Eur J Oral Sci. 2006; 114(5): 409–15.
25. Yoshimichi O, Toshiaki K. Development, migration, and survival of mast cells. Immunol Res. 2006; 34(2): 97-115. [ Links ]
26. Thompson-Snipes L, Dhar V, Bond MW, Mosmann TR, Moore KW, Rennick DM. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med. 1991; 173(2): 507-10. [ Links ]
27. Matsuzawa S, Sakashita K, Kinoshita T, Ito S, Yamashita T, Koike K. IL-9 enhances the growth of human mast cell progenitors under stimulation with stem cell factor. J Immunol. 2003; 170(7): 3461-7. [ Links ]
28. Kanbe N, Kurosawa M, Miyachi Y, Kanbe M, Saito H, Matsuda H. Nerve growth factor prevents apoptosis of cord blood-derived human cultured mast cells synergistically with stem cell factor. Clin Exp Allergy. 2000; 30(8): 1113-20. [ Links ]
29. Bressler RB, Thompson HL, Keffer JM, Metcalfe DD. Inhibition of the growth of IL-3-dependent mast cells from murine bone marrow by recombinant granulocyte macrophage-coloning-stimulating factor. J Immunol. 1989; 143(1): 135-9. [ Links ]
30. Saito H, Ebisawa M, Tachimoto H. Selective growth of human mast cells induced by Steel factor, IL-6 and prostaglandin E2 from cord blood mononuclear cells. J Immunol. 1996; 157(1): 343-50. [ Links ]
 Endereço para correspondência:
Endereço para correspondência:
Kátia Lucy de Melo Maltos, PhD
Av. Antônio Carlos, 6627 – Campus Pampulha
Belo Horizonte - MG, Brazil
CEP: 31270-901
e-mail: kmaltos@ufmg.br
Recebido para publicação: 24/10/11
Enviado para Reformulação: 30/11/11
Aceito para publicação: 03/02/12













