Serviços Personalizados
Artigo
Links relacionados
Compartilhar
Odontologia Clínico-Científica (Online)
versão On-line ISSN 1677-3888
Odontol. Clín.-Cient. (Online) vol.11 no.2 Recife Abr./Jun. 2012
ARTIGO DE REVISÃO / REVIEW ARTICLE
Pharmacogenomics and dental practice: clinical implications and current researches
Farmacogenômica e a prática odontológica: implicações clínicas e pesquisas atuais
Irlan de Almeida FreiresI; Lívia Araújo AlvesI; Ricardo Dias de CastroII
IScientific Initiation Student. Federal University of Paraiba, Paraiba, Brazil
IIDDS, MSc, PhD, Adjunct Professor Department of Clinics and Social Dentistry School of Dentistry. Federal University of Paraiba, Paraiba, Brazil
ABSTRACT
Variations in the response to dental treatments may be due to several factors, including genetic variability. Pharmacogenomics is the application of genomics technology to the development of specific drugs and its relationship with dentistry is a recent area of research. This paper aims to discuss the relationship between pharmacogenomics and dental practice, focusing on clinical implications and current researches. It was used technique of documentation based on literature available at Scielo (Scientific Electronic Library Online) and MEDLINE between 2000 and 2010. Diseases and disorders can be associated with misspellings or genetic mutations. The knowledge of how genetic variation interferes in the response to treatment will allow the development of drugs to be used, for instance, in oral and systemic infection therapy; for the management of oral lesions (e.g. herpes, squamous cell carcinoma), bone resorption (e.g. periodontal diseases); chronic oral and facial pain; for the management of autoimmune and temporomandibular joint disorders. Periodontics, Cariology, Oral Pathology, among other areas, represent a vast field of research yet to be explored. In summary, dentistry begins to show an increasingly close relationship with pharmacogenomics, which may result in the development and improvement of treatment modalities more individualized and potentially more effective.
Keywords: Pharmacogenomics; Dentistry; Phamarcology; Genetic polymorphism.
RESUMO
Variações na resposta aos tratamentos odontológicos ocorrem devido a vários fatores, incluindo variabilidade genética. Farmacogenômica é a aplicação da tecnologia genômica para o desenvolvimento de fármacos específicos, e sua relação com a odontologia é uma área recente de pesquisa. Este trabalho objetiva discutir a relação entre farmacogenômica e a prática odontológica, enfocando as implicações clínicas e pesquisas atuais. Foi utilizada técnica de documentação com base na literatura disponível no Scielo e MEDLINE, entre 2000 e 2010. Doenças e distúrbios podem ser associados a erros de transcrição ou mutações genéticas. O conhecimento de como a variação genética interfere na resposta ao tratamento vai permitir o desenvolvimento de medicamentos a serem utilizados, como na terapia de infecção oral e sistêmica, para o manejo de lesões orais (por exemplo, herpes, carcinoma de células escamosas); reabsorção óssea (por exemplo, doenças periodontais); dores orais e faciais crônicas; doenças autoimunes e distúrbios da articulação temporomandibular. Periodontia, Cariologia, Patologia Oral, entre outras áreas, representam um vasto campo de investigação a ser ainda explorado. Em síntese, a Odontologia vem apresentando uma relação cada vez mais estreita com a farmacogenômica, que pode resultar no desenvolvimento e aperfeiçoamento de modalidades de tratamento mais individualizado e potencialmente mais eficaz.
Descritores: Farmacogenômica; Odontologia; Farmacologia; Polimorfismo genético.
INTRODUCTION
It has long been known that patients treated with the various drugs have variability of response and susceptibility to drug toxicity.1,2 The variations in the response to treatment may be due to several factors such as illness, differences in pharmacokinetics and pharmacodynamics of drugs, environmental factors and genetic factors.1,3 Whereas genetic factors may contribute to the effectiveness and safety of a drug, pharmacogenomics has been recently discussed.1
Pharmacogenomics is the application of genomics technology to the discovery and development of drugs.4,5 It is a field that encompasses the study of genetic polymorphisms that underlie individual differences in drug response.6
According to Shomron7 (2010), this field of the clinical pharmacology studies the contribution of genomes, transcriptomes and proteomes in determining drug-response phenotypes (safety and efficacy). The major goal of pharmacogenomics research is the development of genotype or transcriptome-based predictive tests of drug efficacy or toxicity.
It's well known that therapeutic efficacies and side effect profiles of drugs differ among individuals. Genetic variations in genes encoding components of drug metabolizing enzymes, transporters, primary and secondary targets of metabolites, and downstream pathways are all considered to underlie this difference.3
Molecular dentistry, the Human Genome Project, transcriptomes and proteomes have recently opened vast opportunities for translation of basic science discoveries to oral health care at the chairside and bedside through the intermediary process of clinical research.8
The mouth is a portal of entry as well as a mirror that reflects a wealth of information that can be derived from oral fluids9,10 and tissues. The recent progress of the human and microbial genomes provides unprecedented opportunities to not only understand the molecular basis of oral diseases, but to design and fabricate new generations of diagnostics, therapeutics and biomaterials. In parallel developments, remarkable progress is being made to understand human as well as oral microbial (viral, bacterial and yeast) genetic variations including their respective responses to drug therapies.11
Pharmacogenomics provides new insights into how human genetic variations influence individual drug absorption and allows the development of drugs to be used for oral and systemic infection therapy (viral, bacterial and yeast); for the management of oral lesions (e.g. herpes, squamous cell carcinoma), bone resorption (e.g. periodontal diseases, osteoporosis, osteopetrosis, osteoarthritis); for the management of chronic oral and facial pain (e.g. trigeminal neuralgia), autoimmune disorders (e.g. Sjogren's syndrome with xerostomia); and for the management of temporomandibular joint diseases and disorders.8
Besides that, the knowledge of how genetic variation interferes in the response to treatment will allow the development of drugs that control, for instance, orthodontic tooth movement through the regulation of repair processes and formation of bone and periodontal ligament fibers.12
As can be seen, the completion of human and microbial genome projects will provide a wealth of information that will permit the application of pharmacogenomics – how genetic variations will impact the efficacy of drugs and the diagnosis and treatment of oral diseases.13
Thus, this review aims to discuss the relationship between pharmacogenomics and dental practice, focusing on clinical implications and current researches.
METHODS
It was used an inductive methodological approach and technical documentation based on pre-existing literature on international scientific papers, theses and books, found on the internet and in libraries.
The composition of this paper resulted from research on the databases Pubmed (U.S. National Library of Medicine) – Indexed for Medline and Scielo (Scientific Electronic Library Online) between 2000 and 2010, using the keywords "Pharmacogenomics", "Pharmacogenomics AND Dental Practice," "Genetics AND Dental "and" Polymorphism, Genetic".
Papers from previous years are listed or they are in the bibliography according to their importance for the issue at hand.
LITERATURE REVIEW
• Clinical Implications for Dental Practice
Diseases and disorders can be associated with misspellings or genetic mutations of one or more nucleotides, and these mutations can be caused or induced by infectious mi-Pharmacogenomics and dental practice: clinical implications and current researches Freires IA, et al. crobes, environmental factors such as physical and chemical mutagens, genetic mutations and variations, or (more likely) combinations of these multiple factors.14
Variations or polymorphisms in a single base or nucleotide within the genome (i.e., one of the 3.2 billion bases) may be informative for the diagnosis of a disease.14 The approximately three million different single nucleotide variance or polymorphism (SNPs) are physically distributed throughout the entire genome. SNPs are single nucleotide polymorphisms or one-letter variations in the DNA sequence. These variations in SNPs contribute to differences among individuals. The majority of SNPs may have no deleterious effects; others cause subtle differences in countless characteristics such as tooth size and shape, while others affect the risk for diseases or disorders and are associated with human complex diseases.14,15
Besides that, as the gateway to the body, a constant barrage of invaders — viruses, bacteria, parasites, and fungi — challenges the mouth. Therefore, transmissible infectious diseases, notably dental caries and periodontal disease, predominate among the ills that can compromise oral health.11
Dental caries is a transmissible infectious disease and the bacterial pathogens are transmitted from caregiver to infant during early childhood.11 These microbes form a complex dental plaque of biofilms that adheres to tooth surfaces.11,16 Within these biofilms, mutans streptococci and several other bacteria ferment sugars and other carbohydrates to form lactic and other acids. Repeated cycles of acid generation can result in the microscopic dissolution of minerals in tooth enamel.11,17,18 The initiation and progression of this chronic infectious disease is modulated by genetic variations of the microbial quorum sensing, colonization and acid production within the biofilms, the host genetic variations in enamel matrix composition and structure, the environment such as the presence of fluoride in drinking water, and caregiver as well as infant/toddler/child behavioral factors (frequency and duration of consumption and composition of food and drink choices along with personal oral hygiene).11,19
Mechanical tooth brushing, chemicals as well as immunological (i.e. monoclonal antibodies for passive immunization against microbial antigens related to colonization) methods are used to inhibit or reduce microbial colonization on tooth surfaces. The advances from human and microbial genomics provide many opportunities for targeted therapies such as vaccine developments for children at risk for dental caries.11
Oral microbial infections are also associated with systemic diseases including cardiovascular disease, cerebrovascular disease, low-birth weight, premature babies, osteoarthritis, a number of pulmonary diseases and disorders, and the management of type 1 diabetes.20
According to Cohen and Slavkin21 (2000) one of the major chronic infections is Periodontitis. The virulence of periodontal pathogens includes their role in colonization in subgingival biofilms, synthesis and secretion of cytokines that can directly injure adjacent tissues, and their capacity to invoke inflammatory responses.
There's been shown that some growth factors like IGF (insulin-like growth factor), PDGF (Platelet-derived growth factor) and TGF (Transforming growth factor) and IL-1 (interleukin 1) are associated with inflammatory exudate that occurs in the gingiva and periodontal ligament in patients with periodontitis.22
Karimbux et al.23 (1998) conducted a study in which was verified that chemically modified tetracycline (CMT-1) increased collagen expression in induced periodontal lesions in rats Sprague Dawley. The use of CMT-1 as an adjuvant treatment of periodontal disease is advantageous because it can prevent problems of microbial resistance and retain anti-inflammatory effects of mechanical therapy.
Another considerable disease that affects oral health is candidiasis. Deficiencies in the immune and endocrine systems in children and adults as well as cancer chemotherapy invoke oral candidiasis, which is mainly cause by Candida albicans (the most common fungal pathogen isolated from the oral cavity). The spread of candidiasis to the esophagus or lungs, especially in immunodeficient individuals, can often be life threatening. The near completion of the Candida albicans genome will soon accelerate the identification of innovative antifungal therapies.11
Thus, the completion of the human genome, microbial genomes and functional genomic studies will reveal the hereditability of susceptibility to a variety of infectious diseases (including periodontitis and oral candidiasis); and will elucidate critical cellular pathways associated with the initiation and progression of disease, and may also provide candidate targets for combined drug therapies.24,25 The knowledge of molecules and genes trigger in the development of caries, periodontal disease, cancer, syndromes, TMJ disorders, sleep apnea, and malocclusion will allow the creation of specific individual drugs for caries, periodontal and joint inflammation, sleep apnea, cancer, Sjogren syndrome and malocclusions.12
Birth defects (developmental malformations) appear most commonly as cases of cleft lip with or without cleft palate; facial clefting and/or other craniofacial defects can also be part of complex hereditary diseases or craniofacial syndromes. At this time, hundreds of genetic mutations have been identified that result in facial developmental defects and dental extracellular matrix tissue defects such as enamel (Amelogenesis imperfecta), dentin (Dentinogenesis imperfecta), bone (osteogenesis imperfecta), and cartilage (chondrodysplasia).11
In the field of orthodontics the pharmacogenomic studies can also be very useful in the future. Once the knowledge in biology of tooth movement is still in progress, immunohistochemical techniques have been useful in investigations related to cellular reactions that occur in the periodontal ligament after applying an orthodontic force. Endothelin 1 (ET-1) is a cytokine that is present in the periodontal ligament and in the microvascular bed of orthodontically moved teeth. This peptide also plays an important role in embryogenesis on the following processes: vascular remodeling, regulation of cell proliferation, matrix synthesis, release of growth factors and adhesion molecules and the development of dentofacial anomalies.26
Otero12 (2003) points out that the knowledge about these and other molecules involved in biological response to mechanical force produced by orthodontics will allow, in the future, the control of orthodontic tooth movement by the administration of pharmaceutical molecules that target blood vessels in the periodontal ligament.
Besides that, the study of molecules involved in the growth of teeth, bone and periodontal tissues supports the design of specific drugs to stimulate or inhibit growth in skeletal malocclusions and craniofacial pathologies.12
The opportunities for pharmacogenomics to impact the study of differential gene expression applied to drug discovery and optimization are remarkable. These advances will likely include the discovery of new drug targets, the discovery of new disease and disorder mechanism(s) of the drug, the confirmation of expected action(s) of mechanism of a drug, and the optimal clinical efficacy of the drug or therapeutic for oral health care.11
• Current Researches
The identification of genes responsible for diseases will allow the pharmaceutical industry to synthesize more individual and specific drugs. Gene therapy may be used more reliably and with fewer side effects than those reported today.12
The relationship of dentistry with the field of pharmacogenetics (effects of single genes) or pharmacogenomics (effects of several genes and their interactions) is a recent area of scientific research and, in some respects, hasn't brought major changes from the clinical point of view yet.
However, studies have been already conducted to elucidate aspects of clinical cariology such as identification of cariogenic bacteria in human saliva through PCR (Polymerase Chain Reaction)19; association of polymorphisms with salivary buffer capacity, dental plaque pH, and caries index27; relationship between gene polymorphisms and children's dental fluorosis28, among many others.
Saliva (oral fluid) is an emerging biofluid for non-invasive diagnostics used in the detection of human diseases8,9 as well as for the management of drug therapy, and for a number of forensic applications8. For instance, Aydin29 (2007) points out that specific salivary biomarkers such as glucose, α-amylase, and ghrelin appetite hormone exhibit strong diagnostic potential for diseases such as diabetes.
The ability to monitor health status, disease onset, progression, recurrence and treatment outcome through non-invasive means is highly important to advancing health care management. Saliva is a perfect medium to be explored for personalized individual medicine/dentistry including diagnostics, offering a non-invasive, easy to obtain means for detecting and monitoring diseases.9
In order to develop researches concerning gene polymorphisms investigation and their relation with malfunctions, case-control studies are commonly conducted. In a case-control study patients who have developed a disease are identified and their past exposure to suspected aetiological factors is compared with that of controls or referents who do not have the disease.
Wang28 et al. (2010) conducted a case-control study among children aged 8 to 12 years old, exploring the distribution of ER RsaI genotype (ER gene polymorphism would be associated with bone metabolism and bone mineral density) in the children who lived in the areas with or without high fluoride in drinking water, and investigated the relationship between ER gene RsaI polymorphisms and children's dental fluorosis. They found no correlation between both variables, but suggest that in dental fluorosis perhaps several genes are influencing dental malformations. Therefore, further investigation on other polymorphisms of ER gene and other candidate genes related to calcium-metabolism may be useful.
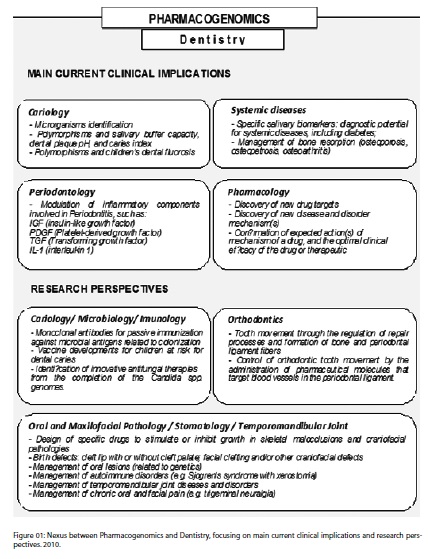
Many areas of Dentistry, especially Periodontics, Cariology, Oral Pathology and Applied Pharmacology, represent a vast field of research to be explored in relation to immunology, modulation of inflammatory processes and development of specific drugs to combat oral pathogenic microrganisms from the decoding of their genomes (Figure 1). However, some matters can hinder this research process, mainly as regards the essays of new drugs.
Until recently, it was difficult to carry out rigorous studies to determine the contribution of pharmacokinetic (eg. metabolism and transport) and/or pharmacodynamic (eg. receptors) factors to interpatient variability in response and the potentially pivotal role that could be played by pharmacogenomics. Since the early part of this century, advances in population pharmacokinetics, objective measures of drug effect and the technology to easily, rapidly and cheaply genotype for any drug metabolising enzyme, transporter and receptor/target, has allowed for valuable insights into explaining why some patients respond poorly, why others experience unacceptable adverse effects necessitating drug withdrawal and why dosage requirements vary substantially between patients.30
CONCLUSIONS
Although individualized therapy remains as a challenge for the future, pharmacogenomics will bring many benefits to public health and to dentistry in particular.
Several areas, including Periodontics, Oral Pathology, Cariology, Orthodontics, among many others, begin to show an increasingly close relationship with pharmacogenomics, which may result (in a not too distant future) in the development and improvement of treatment modalities more individualized and potentially more effective.
Thus, the intersection of biotechnology and genetic information applied to dentistry is the harbinger of the future oral health care.
REFERENCES
1. Shastry BS. Pharmacogenetics and the concept of individualized medicine. Pharmacogenomics J. 2006 ; 6(1):16-21. [ Links ]
2. Relling MV, Hoffman JM. Should Pharmacogenomic studies be required for new drug approval? Clin Pharmacol Ther. 2007; 81:425-8. [ Links ]
3. Kukreti R, Grover S, Gupta M, Sharma A, Sharma S, Bala K et al. Pharmacogenomics and predictive therapy for complex diseases. Genomic Med. 2008 ; 2:341–50.
4. Bumol T, Watanabe A. Genetic information, genomic technologies, and the future of drug discovery. JAMA 2001; 285: 551-5. [ Links ]
5. Rothstein MA, Epps PG. Science and society: ethical and legal implications of pharmacogenomics. Nat Rev Genet. 2001; 2:228-31. [ Links ]
6. Issa AM. Ethical perspectives on pharmacogenomic profiling in the drug development process. Nature Rev Drug Discov. 2002; 1:300-8. [ Links ]
7. Shomron N. MicroRNAs and pharmacogenomics. Pharmacogenomics 2010 11(5):629–32.
8. Slavkin HC. The future of oral health professions: molecular dentistry (an essay). Saudi Dental Journal 2004; 16(1):1-2 [Editorial] [ Links ].
9. Ai J, Smith B, Wong DT. Saliva Ontology: An ontology-based framework for a salivaomics knowledge base. BMC bioinformatics 2010; 11:302. [ Links ]
10. Scott DA, Renaud DE, Krishnasamy S, Meriç P, Buduneli N, Çetinkalp S et al. Diabetes-related molecular signatures in infrared spectra of human saliva. Diabetology & Metabolic Syndrome 2010; 2:48-57. [ Links ]
11. Slavkin HC. Implications of pharmacogenomics in oral health. Pharmacogenomics J. 2002; 2:148-51. [ Links ]
12. Otero LM. La farmacogenómica en la odontología. Pontificia Universidad Javeriana, Bogotá, 2003. Available at: http://recursostic.javeriana.edu.co/doc/farmacoge.pdf [ Links ]
13. Slavkin HC. Applications of pharmacogenomics in general dental practice. Pharmacogenomics 2003; 4(2):163-70. [ Links ]
14. Chambers D. DNA, the double helix: perspective and prospective at forty years. New York: New York Academy of Sciences, 1995. [ Links ]
15. McCarthy JJ, Hilfiker R. The use of single-nucleotide polymorphism maps in pharmacogenomics. Nat Bio. 2000 18:505-08. [ Links ]
16. Gibbons RJ. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989; 68(5):750-60. [ Links ]
17. Jeon J-G, Klein MI, Xiao J, Gregoire S, Rosalen PL, Koo H. Influences of naturally occurring agents in combination with fluoride on gene expression and structural organization of Streptococcus mutans in biofilms. BMC Microbiology 2009; 9:228-38. [ Links ]
18. Wolff MS, Larson C. The cariogenic dental biofilm: good, bad or just something to control? Braz Oral Res. 2009; 23(supl.1): 31-8. [ Links ]
19. Salazar LA, Vasquez C, Almuna A, Oporto G, Santana R, Herrera CL et AL. Detección Molecular de Estreptococos Cariogénicos en Saliva. Int. J. Morphol. 2008; 26(4):951-58. [ Links ]
20. Dasanayake AP. Poor periodontal health of the pregnant woman as a risk for low birth weight. Ann Periodontol. 1998; 13:206-12. [ Links ]
21. Cohen DW, Slavkin HC. Periodontal disease and systemic disease. In: Rose LF, Genco RJ,Cohen DW, Mealey BL (eds). Periodontal medicine. BC Decker: Hamilton, Canada; p.1-10, 2000. [ Links ]
22.Soory M. Targets for steroid hormone mediated actions of periodontal pathogens, Cytokines and therapeutic agents:some implications on tissue turnover in the periodontium. Curr Drug Targets. 2000; 1:309-25. [ Links ]
23. Karimbux NY, Ramamurthy NS, Golub LM, Nishimura I. Semi-synthetic tetracycline doxycycline and a chemically modified tetracycline CMT-1 enhanced collagen expression in induced periodontal lesions of Sprawley Dawley rats. J Periodontol. 1998; 69(1):34-40. [ Links ]
24. IHGSC, International human genome sequencing consortium. Initial sequencing and analysis of the human genome. Nature. 2001; 409:860-921. [ Links ]
25. Peltonen L, McKusick VA (2001). Dissecting human diseases in the post genomic era. Science. 2001; 291:1224-29. [ Links ]
26. Shioma Y, Sasaki T, Shibasaki Y. Histological and inmunohistochemical study of endothelin-1 localization in periodontal ligament during experimental tooth movement. Dent Jap. 1995; 32:75-8. [ Links ]
27. Peres RCR, Camargo G, Mofatto LS, Cortellazzi KL, Santos MCLG, Santos MN et al. Association of polymorphisms in the carbonic anhydrase 6 gene with salivary buffer capacity, dental plaque pH, and caries index in children aged 7–9 years. Pharmacogenomics J. 2010; 10:114-9.
28. Wang G, Ba Y, Yang Y, Ren L, Zhu J, Yin G, et al. ERβ gene RsaI polymorphism and children's dental fluorosis. Life Sci J. 2010; 7(1):51-5.
29. Aydin S. A comparison of ghrelin, glucose, alpha-amylase and protein levels in saliva from diabetics. J Biochem Mol Biol. 2007; 40(1):29-35. [ Links ]
30. Somogyi AA, Hardy J. Role of pharmacogenomics in pain therapy: focus on opioids. Cancer Forum. 2010; 34(2):74-6. [ Links ]
 Endereço para correspondência:
Endereço para correspondência:
Ricardo Dias de Castro
Av. Cajazeiras, 475/102, Coral Gables
Manaíra - João Pessoa Paraíba/Brazil
ZIP CODE: 58038-040
e-mail: ricardodiasdecastro@yahoo.com.br
Recebido para publicação: 26/01/11
Aceito para publicação: 30/03/11













